What Is The Purpose Of Titration In Chemistry . It is commonly used in analytical chemistry to determine the. By this process, the acid or. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration requires three basic components: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The basic process involves adding a. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. A liquid of known molarity or normality,. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time.
from general.chemistrysteps.com
Titration requires three basic components: Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. By this process, the acid or. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to determine the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time.
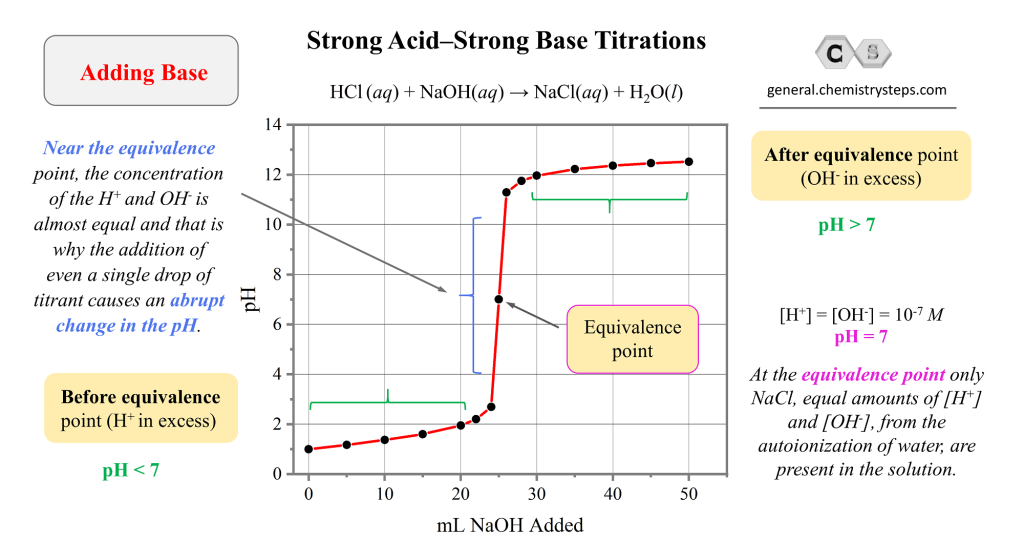
Strong AcidStrong Base Titrations Chemistry Steps
What Is The Purpose Of Titration In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. By this process, the acid or. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration requires three basic components: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A liquid of known molarity or normality,. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. It is commonly used in analytical chemistry to determine the. The basic process involves adding a.
From www.pathwaystochemistry.com
Titrations Introduction Pathways to Chemistry What Is The Purpose Of Titration In Chemistry The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration requires three basic components: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a quantitative analysis to determine the concentration of an unknown. What Is The Purpose Of Titration In Chemistry.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube What Is The Purpose Of Titration In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration requires three basic components: A liquid of known molarity or normality,. By this process, the acid or. It is commonly used in analytical chemistry to determine the. Titration is a quantitative analysis to determine the. What Is The Purpose Of Titration In Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation What Is The Purpose Of Titration In Chemistry It is commonly used in analytical chemistry to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A liquid of known molarity or normality,. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. The purpose. What Is The Purpose Of Titration In Chemistry.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS What Is The Purpose Of Titration In Chemistry By this process, the acid or. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. A liquid of known molarity or. What Is The Purpose Of Titration In Chemistry.
From dokumen.tips
(PPT) Acid Base Titrations Chemistry 12 Chapter 14. Titration A What Is The Purpose Of Titration In Chemistry A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. Titration is the slow addition of one solution. What Is The Purpose Of Titration In Chemistry.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Is The Purpose Of Titration In Chemistry Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. Titration requires three basic components: A titration is a technique where a solution of known concentration is. What Is The Purpose Of Titration In Chemistry.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS What Is The Purpose Of Titration In Chemistry Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A titration is a technique where a solution of known concentration is. What Is The Purpose Of Titration In Chemistry.
From mavink.com
Titration Diagram What Is The Purpose Of Titration In Chemistry By this process, the acid or. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a technique where a solution of known concentration. What Is The Purpose Of Titration In Chemistry.
From www.microlit.com
An Advanced Guide to Titration Microlit What Is The Purpose Of Titration In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A liquid of known molarity or normality,. By this process, the acid or. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. A titration is a technique where a solution of known. What Is The Purpose Of Titration In Chemistry.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 What Is The Purpose Of Titration In Chemistry The basic process involves adding a. It is commonly used in analytical chemistry to determine the. A liquid of known molarity or normality,. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of. What Is The Purpose Of Titration In Chemistry.
From idealpost.co.uk
Purpose And Important Types Of Titration What Is The Purpose Of Titration In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. By this process, the acid or. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. Titration is the. What Is The Purpose Of Titration In Chemistry.
From www.vectorstock.com
Acid base titration experiment and phases Vector Image What Is The Purpose Of Titration In Chemistry The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration. What Is The Purpose Of Titration In Chemistry.
From mavink.com
Titration Picture What Is The Purpose Of Titration In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. Titration requires three basic components: The basic process involves adding a. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of. What Is The Purpose Of Titration In Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Is The Purpose Of Titration In Chemistry By this process, the acid or. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration requires three basic components: Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A. What Is The Purpose Of Titration In Chemistry.
From exytfczfn.blob.core.windows.net
What Is The Purpose Of An AcidBase Titration at Adolph Tilton blog What Is The Purpose Of Titration In Chemistry Titration requires three basic components: Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A liquid of known molarity or normality,. Titration is the slow addition of one solution. What Is The Purpose Of Titration In Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog What Is The Purpose Of Titration In Chemistry The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. It is commonly used in analytical chemistry to determine the. A titration is a technique where a solution of known concentration is used. What Is The Purpose Of Titration In Chemistry.
From mungfali.com
Titration Steps What Is The Purpose Of Titration In Chemistry It is commonly used in analytical chemistry to determine the. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. The purpose. What Is The Purpose Of Titration In Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool What Is The Purpose Of Titration In Chemistry By this process, the acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a quantitative analysis to determine the concentration of. What Is The Purpose Of Titration In Chemistry.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog What Is The Purpose Of Titration In Chemistry A liquid of known molarity or normality,. The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. Titration is the slow addition of one solution of a known concentration (called a titrant) to. What Is The Purpose Of Titration In Chemistry.
From ar.inspiredpencil.com
Titration Setup Diagram What Is The Purpose Of Titration In Chemistry The basic process involves adding a. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. By this process, the acid or. It is commonly used in analytical chemistry to determine the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. What Is The Purpose Of Titration In Chemistry.
From facts.net
8 Captivating Facts About AcidBase Titration What Is The Purpose Of Titration In Chemistry A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration requires three basic components: Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. It is commonly. What Is The Purpose Of Titration In Chemistry.
From joioqtatt.blob.core.windows.net
Types Of Titration Reactions at Alex Ortiz blog What Is The Purpose Of Titration In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the. What Is The Purpose Of Titration In Chemistry.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators What Is The Purpose Of Titration In Chemistry The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The purpose of titration is to determine an unknown concentration in a sample using. What Is The Purpose Of Titration In Chemistry.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog What Is The Purpose Of Titration In Chemistry The basic process involves adding a. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. A titration is a technique where a solution of known concentration. What Is The Purpose Of Titration In Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge What Is The Purpose Of Titration In Chemistry A liquid of known molarity or normality,. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is a quantitative analysis to determine the concentration of an. What Is The Purpose Of Titration In Chemistry.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps What Is The Purpose Of Titration In Chemistry The purpose of titration is to determine an unknown concentration in a sample using an analytical method. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition. What Is The Purpose Of Titration In Chemistry.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube What Is The Purpose Of Titration In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration requires three basic components: Titration, process of chemical analysis. What Is The Purpose Of Titration In Chemistry.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation What Is The Purpose Of Titration In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. By this process, the acid or. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is the slow addition of one solution. What Is The Purpose Of Titration In Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is The Purpose Of Titration In Chemistry It is commonly used in analytical chemistry to determine the. Titration requires three basic components: The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample. What Is The Purpose Of Titration In Chemistry.
From acidsandbasesandmore.weebly.com
Titrations CHEMISTRY 101 What Is The Purpose Of Titration In Chemistry A liquid of known molarity or normality,. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the amount of one. What Is The Purpose Of Titration In Chemistry.
From www.scribd.com
Titration PDF Titration Chemistry What Is The Purpose Of Titration In Chemistry A liquid of known molarity or normality,. It is commonly used in analytical chemistry to determine the. The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known quantity of. The purpose of any titration is. What Is The Purpose Of Titration In Chemistry.
From es.slideshare.net
Tang 07 titrations 2 What Is The Purpose Of Titration In Chemistry The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. The purpose of titration is to determine an unknown concentration in a sample using an analytical method. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration. What Is The Purpose Of Titration In Chemistry.
From joioqtatt.blob.core.windows.net
Types Of Titration Reactions at Alex Ortiz blog What Is The Purpose Of Titration In Chemistry The basic process involves adding a. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration requires three basic components: By this process, the acid or. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance.. What Is The Purpose Of Titration In Chemistry.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs What Is The Purpose Of Titration In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration requires three basic components: The purpose of titration is to determine an unknown concentration in a sample using an analytical method. A titration is a technique where a solution of known concentration is used to determine the concentration of. What Is The Purpose Of Titration In Chemistry.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts What Is The Purpose Of Titration In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. The basic process involves adding a. Titration requires three basic components: The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A liquid of known molarity or normality,.. What Is The Purpose Of Titration In Chemistry.