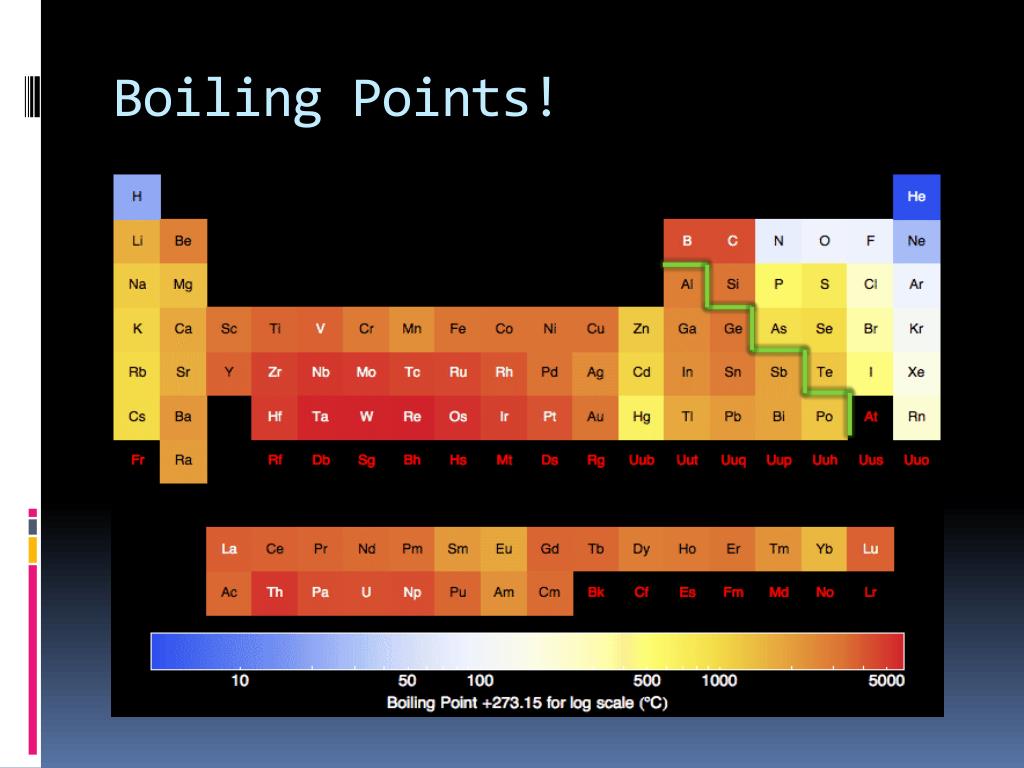
What Is Boiling Point Rise . When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. The change from a liquid phase to. The reason boiling point changes with elevation is because. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Difference between the boiling points. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The temperature at which this. The temperature at which the solvent boils is. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The boiling points of solutions are all higher than that of the pure solvent.
from www.slideserve.com
Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. The change from a liquid phase to. The temperature at which this. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The temperature at which the solvent boils is. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst.
PPT Melting Point and Boiling Point PowerPoint Presentation, free
What Is Boiling Point Rise The temperature at which this. The reason boiling point changes with elevation is because. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The boiling points of solutions are all higher than that of the pure solvent. The temperature at which the solvent boils is. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The temperature at which this. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The change from a liquid phase to. Difference between the boiling points. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst.
From www.youtube.com
Calculating boiling point elevation YouTube What Is Boiling Point Rise The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The temperature at which this. The temperature at which the solvent boils is. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. The reason boiling point. What Is Boiling Point Rise.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is Boiling Point Rise Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The temperature at which this. The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point of water is the. What Is Boiling Point Rise.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Boiling Point Rise For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. The reason boiling point changes with elevation is because. The change from a liquid phase to. Difference between the boiling points. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point elevation. What Is Boiling Point Rise.
From www.jove.com
11369.jpg What Is Boiling Point Rise Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Difference between the boiling points. The reason boiling point changes with elevation is because. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c.. What Is Boiling Point Rise.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is Boiling Point Rise Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure. What Is Boiling Point Rise.
From www.slideserve.com
PPT States, Boiling Point, Melting Point, and Solubility PowerPoint What Is Boiling Point Rise Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The temperature at which this. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Boiling Point Rise.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is Boiling Point Rise Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The temperature at which this. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100. What Is Boiling Point Rise.
From mavink.com
Elevation In Boiling Point Graph What Is Boiling Point Rise Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. Difference between the boiling points. The boiling point of water is the temperature where the liquid’s vapor pressure equals. What Is Boiling Point Rise.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point What Is Boiling Point Rise The reason boiling point changes with elevation is because. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The boiling points of solutions are all higher than that of the pure solvent. Difference between the boiling points. Boiling is the process by which a liquid turns into a vapor. What Is Boiling Point Rise.
From preparatorychemistry.com
pH and Equilibrium What Is Boiling Point Rise Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. Boiling point elevation is the increase in the boiling. What Is Boiling Point Rise.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point What Is Boiling Point Rise The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Difference between the boiling points. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. For example, dissolving salt in water raises the boiling point of water so that it is higher than. What Is Boiling Point Rise.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point What Is Boiling Point Rise Difference between the boiling points. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point elevation occurs when the boiling point of a solution becomes higher than the. What Is Boiling Point Rise.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is Boiling Point Rise The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The change from a liquid phase to. The temperature at which this. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The reason boiling point changes with elevation is because. Boiling point, temperature. What Is Boiling Point Rise.
From inspiritvr.com
Boiling Point Elevation and Freezing Point Depression Study Guide What Is Boiling Point Rise The boiling points of solutions are all higher than that of the pure solvent. Difference between the boiling points. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when. What Is Boiling Point Rise.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free What Is Boiling Point Rise Difference between the boiling points. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The temperature at which the solvent boils is. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling point elevation occurs when the boiling. What Is Boiling Point Rise.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint What Is Boiling Point Rise Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The temperature at which the solvent boils is. Difference between the boiling points. Boiling point, temperature at which the. What Is Boiling Point Rise.
From www.worksheetsplanet.com
What is Boiling Point What Is Boiling Point Rise Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is. What Is Boiling Point Rise.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is Boiling Point Rise Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The temperature at which the solvent boils is. The boiling points of solutions are all higher than that of the pure solvent. Boiling. What Is Boiling Point Rise.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is Boiling Point Rise Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The change from a liquid phase to. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling is the process by which a liquid turns into a vapor. What Is Boiling Point Rise.
From www.slideserve.com
PPT Chapter Introduction Lesson 1 Physical Properties Lesson 2 What Is Boiling Point Rise The reason boiling point changes with elevation is because. The temperature at which the solvent boils is. The temperature at which this. The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, dissolving salt in water raises the boiling point of. What Is Boiling Point Rise.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is Boiling Point Rise Difference between the boiling points. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The change from a liquid phase to. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. Boiling point. What Is Boiling Point Rise.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is Boiling Point Rise When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. Difference between the boiling points. Boiling point elevation occurs when the boiling point of a. What Is Boiling Point Rise.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Boiling Point Rise When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. Difference between the boiling points. The boiling points of solutions are all higher than that of the pure solvent. Boiling is the process by which a liquid turns into a vapor when it is heated. What Is Boiling Point Rise.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Is Boiling Point Rise When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The temperature at which the solvent boils is. The reason boiling point changes with elevation. What Is Boiling Point Rise.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID What Is Boiling Point Rise The temperature at which the solvent boils is. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. For example, dissolving salt in water. What Is Boiling Point Rise.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is Boiling Point Rise The reason boiling point changes with elevation is because. Difference between the boiling points. For example, dissolving salt in water raises the boiling point of water so that it is higher than 100 °c. The temperature at which this. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling. What Is Boiling Point Rise.
From www.youtube.com
Boiling point diagram YouTube What Is Boiling Point Rise Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Difference between the boiling points. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The boiling point of water is the temperature where the liquid’s vapor pressure equals. What Is Boiling Point Rise.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of What Is Boiling Point Rise The boiling points of solutions are all higher than that of the pure solvent. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The change from a liquid phase to. The temperature at which the solvent boils is. Difference between the boiling points. When the vapor pressure inside the bubbles is equal to. What Is Boiling Point Rise.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5694866 What Is Boiling Point Rise The temperature at which the solvent boils is. The change from a liquid phase to. Difference between the boiling points. The boiling points of solutions are all higher than that of the pure solvent. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The boiling point of water is. What Is Boiling Point Rise.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Is Boiling Point Rise Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The temperature at which the solvent boils is. The reason boiling point changes with elevation is because. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point,. What Is Boiling Point Rise.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free What Is Boiling Point Rise The temperature at which this. The reason boiling point changes with elevation is because. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;.. What Is Boiling Point Rise.
From scienceisntscary.wordpress.com
Boiling point Ease Into Science What Is Boiling Point Rise When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Boiling Point Rise.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER What Is Boiling Point Rise The boiling points of solutions are all higher than that of the pure solvent. When the vapor pressure inside the bubbles is equal to the external atmospheric pressure, the bubbles rise to the surface of the liquid and burst. The temperature at which the solvent boils is. The temperature at which this. Boiling point elevation occurs when the boiling point. What Is Boiling Point Rise.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols What Is Boiling Point Rise Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. The temperature at which this. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Boiling Point Rise.
From www.youtube.com
The rise in boiling point of a solution containing `1.8g` glucose in What Is Boiling Point Rise The temperature at which this. Difference between the boiling points. The reason boiling point changes with elevation is because. The change from a liquid phase to. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. What Is Boiling Point Rise.