Copper Gain Lose Electrons . each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. 93 rows this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. according to my book, metals want to lose electrons to achieve noble gas configuration. But at the same time, copper ions in a.
from slidetodoc.com
during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. But at the same time, copper ions in a. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. according to my book, metals want to lose electrons to achieve noble gas configuration. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. 93 rows this table shows the most common charges for atoms of the chemical elements.
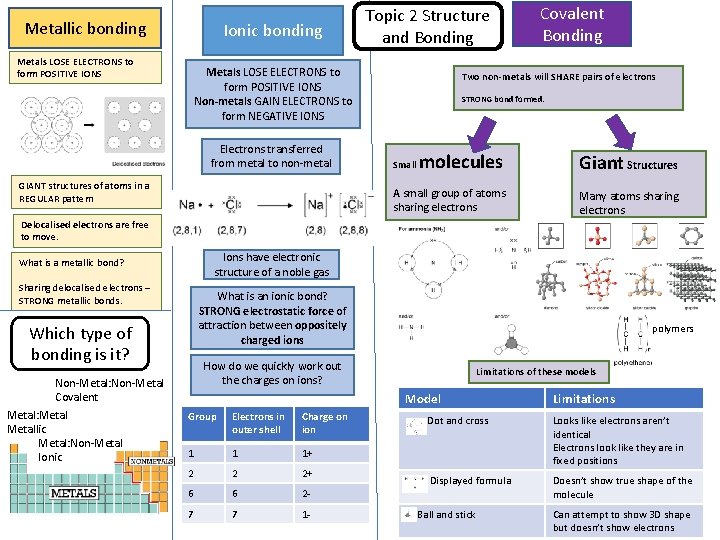
Metallic bonding Metals LOSE ELECTRONS to form POSITIVE
Copper Gain Lose Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. 93 rows this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. according to my book, metals want to lose electrons to achieve noble gas configuration. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. But at the same time, copper ions in a.
From www.teachoo.com
Elements P, Q, R & S have atomic numbers 11, 15, 17 & 18 respectively Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. 93 rows this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance. Copper Gain Lose Electrons.
From physicalsciencetext.weebly.com
8.2 Periodic Trends Physical Science Copper Gain Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. But at the same time, copper ions in a. each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. atoms tend to lose, gain, or share some valance. Copper Gain Lose Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Gain Lose Electrons But at the same time, copper ions in a. according to my book, metals want to lose electrons to achieve noble gas configuration. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. during the formation of some compounds, atoms gain or lose electrons, and form electrically. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Nomenclature of Compounds PowerPoint Presentation, free Copper Gain Lose Electrons atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. 93 rows this table shows the most common charges for atoms of the chemical elements. according. Copper Gain Lose Electrons.
From www.youtube.com
Why Metals Lose Electrons and NonMetals Gain Electrons Chemistry of Copper Gain Lose Electrons But at the same time, copper ions in a. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. according to my book, metals want to lose electrons to achieve noble gas configuration. each object has a certain value that defines whether it will lose electrons when rubbing against. Copper Gain Lose Electrons.
From www.numerade.com
SOLVED In the first step ofthe lab Cu (s) 4 HNOz (aq) + Cu(NOz)z Copper Gain Lose Electrons But at the same time, copper ions in a. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. 93 rows this table shows the most common charges for atoms of the chemical elements. In general, metals will lose electrons to become a positive cation and nonmetals will. Copper Gain Lose Electrons.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Gain Lose Electrons during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. according to my book, metals want to lose electrons to achieve noble gas configuration. In general, metals will lose electrons to. Copper Gain Lose Electrons.
From www.numerade.com
SOLVED Examining your labeled periodic table, which of the following Copper Gain Lose Electrons the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. 93 rows. Copper Gain Lose Electrons.
From dxouwsswb.blob.core.windows.net
Does Copper Lose Electrons Easily at Santo Weaver blog Copper Gain Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a.. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Atoms in Combination The Chemical Bond PowerPoint Presentation Copper Gain Lose Electrons But at the same time, copper ions in a. each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. according to my book, metals want to lose electrons to achieve noble gas configuration. during the formation of some compounds, atoms gain or lose. Copper Gain Lose Electrons.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Gain Lose Electrons each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the valency of an element indicates the number of electrons it can gain,. Copper Gain Lose Electrons.
From slideplayer.com
Periodic Table. ppt download Copper Gain Lose Electrons But at the same time, copper ions in a. according to my book, metals want to lose electrons to achieve noble gas configuration. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a.. Copper Gain Lose Electrons.
From www.slideserve.com
PPT The Chemical Bond PowerPoint Presentation, free download ID5702058 Copper Gain Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. according to my book, metals want to lose electrons to achieve noble gas configuration. each object has a certain value that defines whether it will. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609 Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. 93 rows this table shows the most common charges for atoms of the chemical elements. the valency of an element indicates the number of electrons it. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Bonding Between Atoms PowerPoint Presentation, free download ID Copper Gain Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. But at the same time, copper ions in a. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Metals, Nonmetals, and Movement of Electrons PowerPoint Copper Gain Lose Electrons atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. 93 rows this table shows the most common charges for atoms of the chemical elements. In general, metals will lose electrons. Copper Gain Lose Electrons.
From dxouwsswb.blob.core.windows.net
Does Copper Lose Electrons Easily at Santo Weaver blog Copper Gain Lose Electrons during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. according to my book, metals want to lose electrons to achieve noble gas configuration. But at the. Copper Gain Lose Electrons.
From slideplayer.com
Electrochemistry Movement of Electrons Chemistry of Metal Reactions Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. during the formation of. Copper Gain Lose Electrons.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Copper Gain Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. according to my book, metals want to lose electrons to achieve noble gas configuration. In general, metals will lose electrons to become a positive cation and. Copper Gain Lose Electrons.
From slideplayer.com
Periodic Trends. ppt download Copper Gain Lose Electrons during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. . Copper Gain Lose Electrons.
From www.doubtnut.com
copper ions lose electrons to neutral atoms Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. during the formation of. Copper Gain Lose Electrons.
From slidetodoc.com
metals lose valence electrons form cation ion nonmetals Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. But at the same time, copper ions in a. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. each object has a certain value that defines whether it will lose electrons when rubbing against an object. Copper Gain Lose Electrons.
From www.gauthmath.com
Solved 4. When are anions formed? when nonmetals gain electrons when Copper Gain Lose Electrons But at the same time, copper ions in a. according to my book, metals want to lose electrons to achieve noble gas configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. In general, metals will lose electrons to become a positive cation and nonmetals will gain. Copper Gain Lose Electrons.
From www.chegg.com
Solved METALS LOSE ELECTRONS and NONMETALS GAIN ELECTRONS. Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. 93 rows this table shows the most common charges for atoms of the chemical elements. the valency of an element indicates the. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID6788469 Copper Gain Lose Electrons each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. 93 rows this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of. Copper Gain Lose Electrons.
From slideplayer.com
Photo by Ra’ike Ionic Compounds. ppt download Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. 93 rows this table shows the most common charges for atoms of the chemical elements. In general, metals will lose electrons to become. Copper Gain Lose Electrons.
From slideplayer.com
Chapter 3 Molecules, compounds, and chemical equations ppt download Copper Gain Lose Electrons each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. 93 rows this table shows the most common charges for atoms of the chemical elements. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. In general,. Copper Gain Lose Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Copper Have? Copper Gain Lose Electrons the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. 93 rows this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. according. Copper Gain Lose Electrons.
From www.slideserve.com
PPT THE NATURE OF MATERIALS PowerPoint Presentation, free download Copper Gain Lose Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. 93 rows this table shows the most common charges for atoms of the chemical elements. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. during the formation. Copper Gain Lose Electrons.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Copper Gain Lose Electrons each object has a certain value that defines whether it will lose electrons when rubbing against an object with a higher value, and vice. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. according to my book, metals want to lose electrons to achieve noble gas. Copper Gain Lose Electrons.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Copper Gain Lose Electrons But at the same time, copper ions in a. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. according to my book, metals want to lose electrons to achieve noble. Copper Gain Lose Electrons.
From slidetodoc.com
Metallic bonding Metals LOSE ELECTRONS to form POSITIVE Copper Gain Lose Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. But at the same time, copper ions in a. during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles. according to my book, metals want to lose electrons to achieve noble gas configuration.. Copper Gain Lose Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Copper Gain Lose Electrons In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the. Copper Gain Lose Electrons.
From www.stonecoldhands.com
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge Copper Gain Lose Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. But at the same. Copper Gain Lose Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper II Easy & Quick YouTube Copper Gain Lose Electrons according to my book, metals want to lose electrons to achieve noble gas configuration. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the valency of an element indicates the number of electrons it can gain, lose, or share to form chemical compounds. But at the. Copper Gain Lose Electrons.