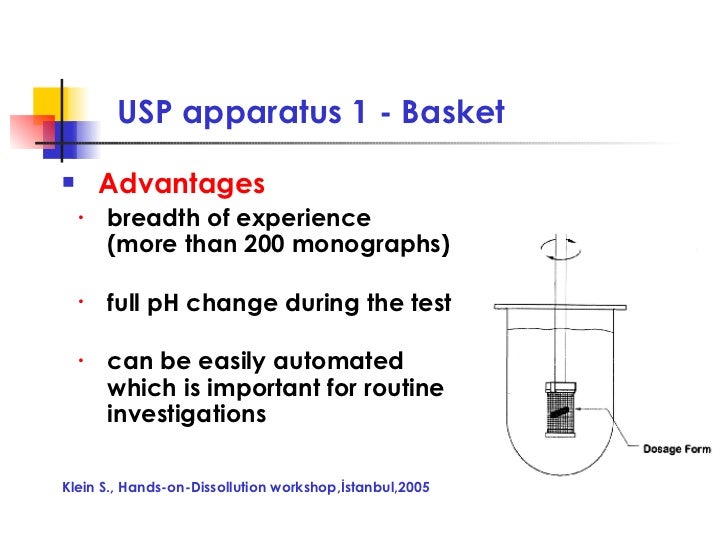
Why Use Basket In Dissolution . It comprises borosilicate glass and holds a capacity of up to 1000 ml. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc.
from www.slideshare.net
in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. It comprises borosilicate glass and holds a capacity of up to 1000 ml. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium.
The role of dissolution in the demonstration of bioequivalence
Why Use Basket In Dissolution for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. It comprises borosilicate glass and holds a capacity of up to 1000 ml. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft.
From www.genetec.se
Stainless Steel Dissolution Baskets 40 Mesh Why Use Basket In Dissolution A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for. Why Use Basket In Dissolution.
From www.slideserve.com
PPT Dissolution and drug release testing PowerPoint Presentation Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. It comprises borosilicate glass and holds a capacity of. Why Use Basket In Dissolution.
From meshfiltro.com
Dissolution basket and Dissolution Sinker Manufacturer Bluslot Why Use Basket In Dissolution in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. the dissolution test in. Why Use Basket In Dissolution.
From www.indiamart.com
8 Basket Dissolution Apparatus at Rs 1500 Dissolution Apparatus in Why Use Basket In Dissolution A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. It comprises borosilicate glass and holds a capacity of up to 1000 ml. . Why Use Basket In Dissolution.
From uspbpep.com
General Chapters DISSOLUTION Why Use Basket In Dissolution for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. It comprises borosilicate glass and holds a capacity of up to 1000 ml. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. dissolution testing provides critical information to support the realisation. Why Use Basket In Dissolution.
From www.slideshare.net
The role of dissolution in the demonstration of bioequivalence Why Use Basket In Dissolution for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. dissolution. Why Use Basket In Dissolution.
From meshfiltro.com
Dissolution basket and Dissolution Sinker Manufacturer Bluslot Why Use Basket In Dissolution It comprises borosilicate glass and holds a capacity of up to 1000 ml. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product. Why Use Basket In Dissolution.
From www.pinterest.com
dissolution basket Dissolution basket method is different from the cup Why Use Basket In Dissolution dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. the dissolution test in a usp drug product monograph helps evaluate. Why Use Basket In Dissolution.
From riggtek.de
RIGGTEK GmbH, Pharmaceutical Dissolution Testing Instruments and Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. A paddle, rotating basket, and/or shaft combination. Why Use Basket In Dissolution.
From meshfiltro.com
Dissolution basket and Dissolution Sinker Manufacturer Bluslot Why Use Basket In Dissolution A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. It comprises borosilicate glass and holds a capacity of up to 1000 ml. . Why Use Basket In Dissolution.
From www.researchgate.net
In vitro drug dissolution profile of diclofenac diethylamine from Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. dissolution testing. Why Use Basket In Dissolution.
From www.youtube.com
Automated Dissolution Basket Test YouTube Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. It comprises. Why Use Basket In Dissolution.
From courses.lumenlearning.com
The Dissolution Process Chemistry Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. It comprises borosilicate glass and holds a capacity of up to 1000 ml. the dissolution test in a. Why Use Basket In Dissolution.
From www.labhut.com
Dabigatran Etexilate Mesylate Dissolution Basket Why Use Basket In Dissolution It comprises borosilicate glass and holds a capacity of up to 1000 ml. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing. Why Use Basket In Dissolution.
From www.slideserve.com
PPT Dissolution PowerPoint Presentation ID266150 Why Use Basket In Dissolution dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. It comprises borosilicate glass and holds a capacity of. Why Use Basket In Dissolution.
From www.labhut.com
Dissolution Basket Tool for Easy Handling Why Use Basket In Dissolution It comprises borosilicate glass and holds a capacity of up to 1000 ml. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. dissolution testing. Why Use Basket In Dissolution.
From www.agilent.com
Dissolution Paddles, Baskets and Shafts Agilent Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of. Why Use Basket In Dissolution.
From www.labhut.com
Dissolution Baskets Holder for 12 Baskets of any Brand. Why Use Basket In Dissolution It comprises borosilicate glass and holds a capacity of up to 1000 ml. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. for example, it is commonly. Why Use Basket In Dissolution.
From www.pinterest.com
Dissolution Basket Product Dissolution basket Material Stainless Why Use Basket In Dissolution for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. dissolution. Why Use Basket In Dissolution.
From www.slideshare.net
The role of dissolution in the demonstration of bioequivalence Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. dissolution testing provides critical. Why Use Basket In Dissolution.
From slideplayer.com
Design and Calibration of a Dissolution Test Equipment Training Why Use Basket In Dissolution dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. A paddle, rotating basket, and/or shaft combination. Why Use Basket In Dissolution.
From riggtek.de
RIGGTEK GmbH, Pharmaceutical Dissolution Testing Instruments and Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. in the basket dissolution method, a different apparatus is placed at the end of the. Why Use Basket In Dissolution.
From www.labhut.com
122100, 40 Mesh Stainless Steel Dissolution Basket for Agilent / VanKel Why Use Basket In Dissolution dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. a dissolution test uses an apparatus with specific test conditions in. Why Use Basket In Dissolution.
From www.slideshare.net
Basic bioequivalence Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product.. Why Use Basket In Dissolution.
From www.slideserve.com
PPT Dissolution PowerPoint Presentation, free download ID9639454 Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. It comprises borosilicate glass and holds a capacity of up to 1000 ml. in the. Why Use Basket In Dissolution.
From meshfiltro.com
Dissolution basket and Dissolution Sinker Manufacturer Bluslot Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug. Why Use Basket In Dissolution.
From knowledgeofpharma.blogspot.com
Dissolution Apparatus Apparatus 1 & 2 Why Use Basket In Dissolution the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. It comprises borosilicate glass and holds a capacity of up to 1000 ml. a dissolution test uses an apparatus with. Why Use Basket In Dissolution.
From www.researchgate.net
(a) Dissolution basket (left panel) and details of the threepronged Why Use Basket In Dissolution A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for. Why Use Basket In Dissolution.
From www.genetec.se
Stainless Steel Dissolution Baskets 20 Mesh Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. for example, it is commonly suggested that the basket apparatus may be preferred for products that may float. Why Use Basket In Dissolution.
From www.genetec.se
Stainless Steel Dissolution Baskets 100 Mesh Dissolution Why Use Basket In Dissolution It comprises borosilicate glass and holds a capacity of up to 1000 ml. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. a dissolution test uses an apparatus with specific test conditions in combination. Why Use Basket In Dissolution.
From www.johnsonwedgewire.com
Stainless Steel Dissolution Baskets 40 Mesh Why Use Basket In Dissolution for example, it is commonly suggested that the basket apparatus may be preferred for products that may float in the. It comprises borosilicate glass and holds a capacity of up to 1000 ml. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. a dissolution test uses an apparatus with. Why Use Basket In Dissolution.
From www.jaytee.com
Sotax 100 Mesh Clip Style Dissolution Basket Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of. Why Use Basket In Dissolution.
From meshfiltro.com
Dissolution basket and Dissolution Sinker Manufacturer Bluslot Why Use Basket In Dissolution It comprises borosilicate glass and holds a capacity of up to 1000 ml. in the basket dissolution method, a different apparatus is placed at the end of the rotating shaft. a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. the dissolution test in a. Why Use Basket In Dissolution.
From www.researchgate.net
GSE capsule residues within the dissolution basket after 2 h Why Use Basket In Dissolution dissolution testing provides critical information to support the realisation of drug release goals, for comparing the performance of different drug substances, for bioequivalence (be) testing and for product qc. It comprises borosilicate glass and holds a capacity of up to 1000 ml. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. . Why Use Basket In Dissolution.
From www.labhut.com
Dissolution Basket Tool for Easy Handling Why Use Basket In Dissolution a dissolution test uses an apparatus with specific test conditions in combination with acceptance criteria to evaluate the performance of the product. A paddle, rotating basket, and/or shaft combination used to promote the movement of the dissolution medium. the dissolution test in a usp drug product monograph helps evaluate the performance of a drug product (article) and indicates.. Why Use Basket In Dissolution.