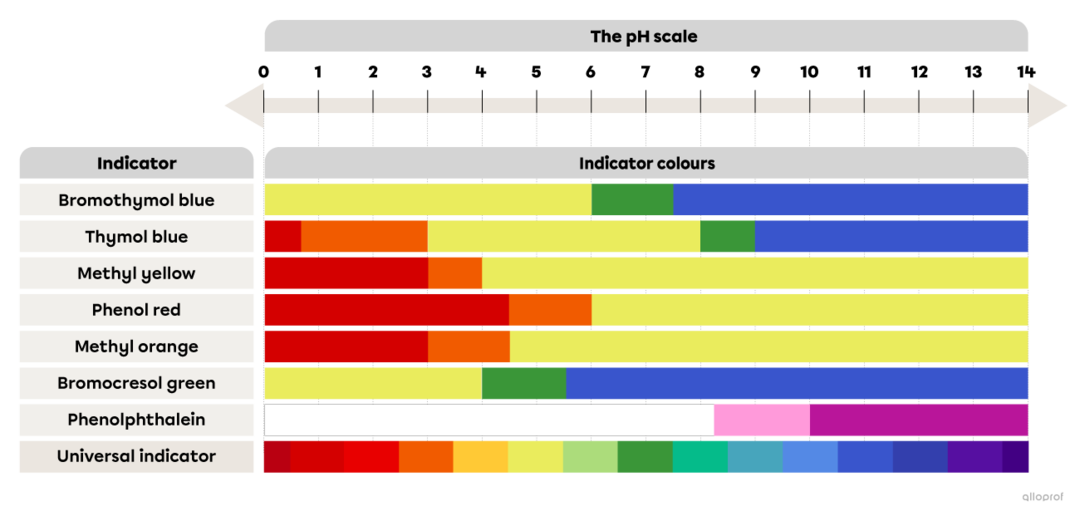
How Do Acid Base Indicators Change Color . A variety of indicators change color at various ph levels. How does chemistry turn this liquid from clear to deep magenta? They are usually weak acids or bases, but their conjugate base or acid. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. How does equilibrium factor into acid/base indicators, and choosing the right indicator. Methyl orange or phenolphthalein would be less. Indicators are substances whose solutions change color due to changes in ph. This is a charge of common indicators, an. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of.
from www.alloprof.qc.ca
They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. How does equilibrium factor into acid/base indicators, and choosing the right indicator. They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances whose solutions change color due to changes in ph. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. Methyl orange or phenolphthalein would be less. How does chemistry turn this liquid from clear to deep magenta? A variety of indicators change color at various ph levels.
The pH Scale and AcidBase Indicators Secondaire Alloprof
How Do Acid Base Indicators Change Color A variety of indicators change color at various ph levels. Methyl orange or phenolphthalein would be less. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are usually weak acids or bases, but their conjugate base or acid. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. This is a charge of common indicators, an. A variety of indicators change color at various ph levels. How does chemistry turn this liquid from clear to deep magenta? How does equilibrium factor into acid/base indicators, and choosing the right indicator. Indicators are substances whose solutions change color due to changes in ph. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7.
From www.chemistry4students.com
Chemistry 4 Students Acid Base indicators How Do Acid Base Indicators Change Color Methyl orange or phenolphthalein would be less. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. How does chemistry turn this liquid from clear to deep magenta? They are usually weak acids or bases, but their. How Do Acid Base Indicators Change Color.
From slideplayer.com
Acids Sour taste Change the color of acidbase indicators ppt download How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Methyl orange. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 How Do Acid Base Indicators Change Color Acid and base indicators are usually used to detect the endpoint of acid and base titrations. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. How does equilibrium factor into acid/base indicators, and choosing the right indicator. This is a. How Do Acid Base Indicators Change Color.
From fyocggitt.blob.core.windows.net
AcidBase Indicators Change Color At Their Ph Endpoint at Billy Glass blog How Do Acid Base Indicators Change Color They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Indicators are substances whose solutions change color due to changes in ph. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. This is a charge of common indicators, an. A variety of indicators change color at. How Do Acid Base Indicators Change Color.
From chimactiv.agroparistech.fr
Chimactiv Interactive numerical educational resources for the How Do Acid Base Indicators Change Color They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. How does equilibrium factor into acid/base indicators, and choosing the right indicator. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. In an acidic solution, the colour indicator will exist in its conjugate acid form (one. How Do Acid Base Indicators Change Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. How does chemistry turn this liquid from clear to deep magenta? A variety of indicators change color at various ph levels. This is a charge of common indicators, an. Methyl orange. How Do Acid Base Indicators Change Color.
From www.youtube.com
AcidBase indicator or pH indicator II Why does indicator change colour How Do Acid Base Indicators Change Color How does chemistry turn this liquid from clear to deep magenta? They are usually weak acids or bases, but their conjugate base or acid. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. Indicators are substances whose solutions change color due to changes in ph. The litmus colour change happens over an unusually. How Do Acid Base Indicators Change Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Do Acid Base Indicators Change Color They are usually weak acids or bases, but their conjugate base or acid. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. How does equilibrium factor into acid/base indicators, and choosing the right indicator. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis. How Do Acid Base Indicators Change Color.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ How Do Acid Base Indicators Change Color Indicators are substances whose solutions change color due to changes in ph. This is a charge of common indicators, an. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. How does chemistry turn this liquid from clear to deep magenta? How does equilibrium factor into acid/base indicators, and choosing the right indicator.. How Do Acid Base Indicators Change Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Do Acid Base Indicators Change Color The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. Indicators are substances whose solutions change color due to changes in ph. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. A variety. How Do Acid Base Indicators Change Color.
From sites.google.com
3 IB Chem 11 HL Whitehair Duarte How Do Acid Base Indicators Change Color The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. How does chemistry turn this liquid from clear to deep magenta? This is a charge of common indicators, an. Acid and base indicators are usually used to detect the endpoint of. How Do Acid Base Indicators Change Color.
From nigerianscholars.com
AcidBase Indicators AcidBase Equilibria How Do Acid Base Indicators Change Color They are usually weak acids or bases, but their conjugate base or acid. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. How does chemistry turn this liquid from clear to deep magenta? How does equilibrium factor into acid/base indicators,. How Do Acid Base Indicators Change Color.
From fyosvzfhj.blob.core.windows.net
Acid Base Indicators Change Color at Phillips blog How Do Acid Base Indicators Change Color Methyl orange or phenolphthalein would be less. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. This is a charge of common indicators, an. How does equilibrium factor into acid/base indicators, and choosing the right indicator. They are usually weak. How Do Acid Base Indicators Change Color.
From slideplayer.com
PART 1 Acid/Base Theory & Properties ppt download How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. A variety of indicators change color at various ph levels. How does chemistry turn this liquid from clear to deep magenta? They are usually weak acids or bases, but their conjugate. How Do Acid Base Indicators Change Color.
From www.alloprof.qc.ca
The pH Scale and AcidBase Indicators Secondaire Alloprof How Do Acid Base Indicators Change Color They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. How does chemistry turn this liquid from clear to deep magenta? This is a. How Do Acid Base Indicators Change Color.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts How Do Acid Base Indicators Change Color How does equilibrium factor into acid/base indicators, and choosing the right indicator. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. This is a charge of common indicators, an. Methyl orange or phenolphthalein would be less.. How Do Acid Base Indicators Change Color.
From fyocggitt.blob.core.windows.net
AcidBase Indicators Change Color At Their Ph Endpoint at Billy Glass blog How Do Acid Base Indicators Change Color How does equilibrium factor into acid/base indicators, and choosing the right indicator. This is a charge of common indicators, an. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its. How Do Acid Base Indicators Change Color.
From knowledge.carolina.com
AcidBase Indicators Carolina Knowledge Center How Do Acid Base Indicators Change Color They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Indicators are substances whose solutions change color due to changes in ph. How does chemistry turn this liquid from clear to deep magenta? Methyl orange or phenolphthalein would be less. Acid and base indicators are usually used to detect the endpoint of acid. How Do Acid Base Indicators Change Color.
From fyosvzfhj.blob.core.windows.net
Acid Base Indicators Change Color at Phillips blog How Do Acid Base Indicators Change Color Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Methyl orange or phenolphthalein would be less. This is a charge of common indicators, an. They are typically weak. How Do Acid Base Indicators Change Color.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Methyl orange or phenolphthalein would be less. The litmus colour change happens over an. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT PROPERTIES OF ACIDS & BASES PowerPoint Presentation, free How Do Acid Base Indicators Change Color Methyl orange or phenolphthalein would be less. How does equilibrium factor into acid/base indicators, and choosing the right indicator. How does chemistry turn this liquid from clear to deep magenta? Acid and base indicators are usually used to detect the endpoint of acid and base titrations. Indicators are substances whose solutions change color due to changes in ph. They are. How Do Acid Base Indicators Change Color.
From www.howtopronounce.com
Take the free online AcidBase Indicators Color Change! Chemistry How Do Acid Base Indicators Change Color How does chemistry turn this liquid from clear to deep magenta? Methyl orange or phenolphthalein would be less. How does equilibrium factor into acid/base indicators, and choosing the right indicator. A variety of indicators change color at various ph levels. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. In an acidic solution,. How Do Acid Base Indicators Change Color.
From fyosvzfhj.blob.core.windows.net
Acid Base Indicators Change Color at Phillips blog How Do Acid Base Indicators Change Color How does chemistry turn this liquid from clear to deep magenta? They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. How does equilibrium factor into acid/base indicators, and choosing the right indicator. A variety of indicators change color at various ph levels. In an acidic solution, the colour indicator will exist in. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 How Do Acid Base Indicators Change Color Methyl orange or phenolphthalein would be less. The litmus colour change happens over an unusually wide range, but it is useful for detecting acids and alkalis in the lab because it changes colour around ph 7. They are usually weak acids or bases, but their conjugate base or acid. A variety of indicators change color at various ph levels. This. How Do Acid Base Indicators Change Color.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic How Do Acid Base Indicators Change Color Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. How does chemistry turn this liquid. How Do Acid Base Indicators Change Color.
From fyocggitt.blob.core.windows.net
AcidBase Indicators Change Color At Their Ph Endpoint at Billy Glass blog How Do Acid Base Indicators Change Color Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. They are usually weak acids or bases, but their conjugate base or acid. A variety of indicators change color. How Do Acid Base Indicators Change Color.
From study.com
Acidic, Basic & Neutral Solutions Overview, pH Scale & Uses Lesson How Do Acid Base Indicators Change Color This is a charge of common indicators, an. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. How does chemistry turn this liquid from clear to deep magenta? How does equilibrium factor into acid/base indicators, and choosing the right indicator.. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID How Do Acid Base Indicators Change Color This is a charge of common indicators, an. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Methyl orange or phenolphthalein would be less. Indicators are substances whose solutions change color due to changes in ph. A variety of indicators. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Methyl orange or phenolphthalein would be less. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. The litmus colour change happens over an unusually. How Do Acid Base Indicators Change Color.
From fyocggitt.blob.core.windows.net
AcidBase Indicators Change Color At Their Ph Endpoint at Billy Glass blog How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Acid and base indicators are usually used to detect the endpoint of acid and base titrations. They are usually weak acids or bases, but their conjugate base or acid. Methyl orange. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID How Do Acid Base Indicators Change Color They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. A variety of indicators change color at various ph levels. Methyl orange or phenolphthalein would be less. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is. How Do Acid Base Indicators Change Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo How Do Acid Base Indicators Change Color This is a charge of common indicators, an. A variety of indicators change color at various ph levels. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. The litmus colour change happens over an unusually wide range, but it is. How Do Acid Base Indicators Change Color.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry How Do Acid Base Indicators Change Color They are usually weak acids or bases, but their conjugate base or acid. Methyl orange or phenolphthalein would be less. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Indicators are substances whose solutions change color due to changes in. How Do Acid Base Indicators Change Color.
From www.slideserve.com
PPT Chapter 15 AcidBase Titrations & pH PowerPoint Presentation ID How Do Acid Base Indicators Change Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. A variety of indicators change color at various ph levels. This is a charge of common indicators, an. The litmus colour change happens over an unusually wide range, but it is. How Do Acid Base Indicators Change Color.
From davidswart.wordpress.com
Acids and Bases The Art of Science How Do Acid Base Indicators Change Color They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. How does equilibrium factor into acid/base indicators, and choosing the right indicator. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Methyl orange. How Do Acid Base Indicators Change Color.