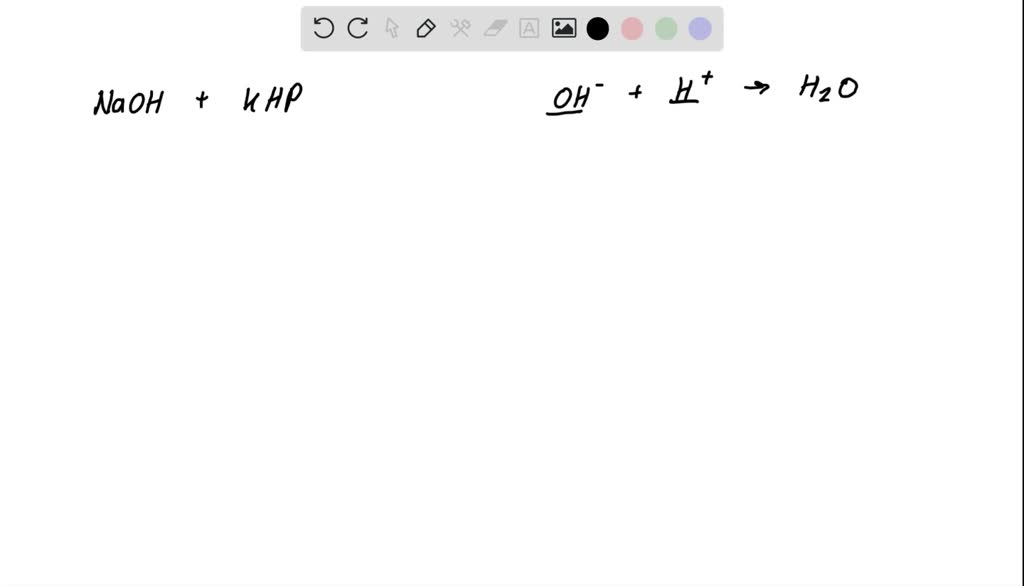
Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation . Molarity of naoh = (mol khp)/ (v naoh used for titration): Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Potassium hydrogen phthalate and sodium hydroxide react as follows: Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Report the determined concentration of the. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using.
from www.numerade.com
One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Report the determined concentration of the. Molarity of naoh = (mol khp)/ (v naoh used for titration): Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Potassium hydrogen phthalate and sodium hydroxide react as follows: To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic.
SOLVED The concentration of a certain sodium hydroxide solution was
Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Potassium hydrogen phthalate and sodium hydroxide react as follows: Report the determined concentration of the. Molarity of naoh = (mol khp)/ (v naoh used for titration): To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret.
From www.numerade.com
SOLVEDSodium hydroxide solution is usually standardized by titrating a Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Report the determined concentration of the. Molarity of naoh = (mol khp)/ (v naoh used for titration): Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Potassium hydrogen phthalate and sodium hydroxide react as follows: Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. To standardize a sodium hydroxide. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved 2. For the above titration of potassium hydrogen Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Report the determined concentration. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved 1. The reaction between KHP (potassium hydrogen Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Report the determined concentration of the. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Potassium hydrogen phthalate and sodium hydroxide react as follows: Molarity of naoh = (mol khp)/ (v. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED A solution of sodium hydroxide (NaOH) was standardized against Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Molarity of naoh = (mol khp)/ (v naoh used for titration): An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED Question 3 What is the KHP NaOH mole ratio for the reaction Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Khc8h4o4 + naoh →. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED In the first part of this lab. you will standardize a sodium Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Report the determined concentration of the. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.studypool.com
SOLUTION Objective to determine the concentration of a solution of Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Molarity of naoh = (mol khp)/ (v naoh used for titration): An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Potassium hydrogen phthalate and sodium hydroxide react as follows: Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED The COMPLETE REACTION Equation UNBALANCED EQUATION First Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Potassium hydrogen phthalate and sodium hydroxide react as follows: One such compound is potassium hydrogen. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED A 25.00 mL portion of 0.0500M potassium hydrogen phthalate is Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Molarity of naoh = (mol khp)/ (v naoh used for titration): To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Potassium hydrogen phthalate and sodium hydroxide react as follows: Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved 5. Determine the mass of potassium hydrogen phthalate Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Potassium hydrogen phthalate and sodium hydroxide react as follows: Molarity of naoh = (mol khp)/ (v naoh used for titration): Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Standardize a sodium hydroxide (naoh) solution. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVEDBonus Question NaOH was standardized by titration against a Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Potassium hydrogen phthalate and sodium hydroxide react as follows: An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). To standardize a solution of sodium hydroxide. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED Experiment Standardization of sodium hydroxide stock solution Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Potassium hydrogen phthalate and sodium hydroxide react as follows: Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. To standardize a sodium hydroxide. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
⏩SOLVEDDerive the following equation for the titration of potassium Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Molarity of naoh = (mol khp)/ (v naoh used for titration): Report the determined concentration of the. Standardize a sodium hydroxide. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED A solution of sodium hydroxide (NaOH) was standardized against Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.gauthmath.com
Solved Part 1 The Reaction Between Sodium Hydroxide And Potassium Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Molarity of naoh = (mol khp)/ (v naoh used for titration): Potassium hydrogen phthalate. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED Potassium hydrogen phthalate (KHP) is primary standard. Explain Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Report the determined concentration of the. Potassium hydrogen phthalate and sodium hydroxide react as follows: Molarity of naoh = (mol khp)/ (v naoh used for titration): One such compound is potassium. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED The following data were collected during the titration of a Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Potassium hydrogen phthalate and sodium hydroxide react as follows: One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Report the determined concentration of the. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. To standardize a solution of sodium. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From solvedlib.com
Potassium hydrogen phthalate (KHP) reacts with sodium… SolvedLib Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Potassium hydrogen phthalate and sodium hydroxide react as follows: Report the determined concentration of the. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Molarity of naoh = (mol khp)/ (v naoh used for titration):. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED Calculating the Concentration of a Sodium Hydroxide Solution Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.echemi.com
What is the chemical reaction when potassium hydrogen phthalate and Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Molarity of naoh = (mol khp)/ (v naoh used for titration): Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved Balanced molecular equation for the reaction of Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Potassium hydrogen phthalate and sodium hydroxide react as follows: Report the determined concentration of the. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.scribd.com
Laboratory Plan 1 Standardization of Sodium Hydroxide (Naoh) With Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. An aqueous solution of sodium hydroxide (naoh) was standardized. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED A student titrates potassium hydrogen phthalate ("KHP" C8H5KO4 Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Report the determined concentration of the. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED The concentration of a certain sodium hydroxide solution was Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Report the determined concentration of the. Molarity of naoh = (mol khp)/ (v naoh used for titration): Potassium hydrogen phthalate and sodium hydroxide react as follows: Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.congress-intercultural.eu
Solved Sodium Hydroxide Reacts With Potassium Hydrogen, 49 OFF Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Report the determined concentration of the. Molarity of naoh = (mol khp)/ (v naoh used for titration): To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Standardize a sodium hydroxide. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From dxopmiczw.blob.core.windows.net
How To Do Titration Calculations A Level Chemistry at Lorraine Nix blog Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Molarity of naoh = (mol khp)/ (v naoh used for titration): Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Report the determined. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED 'Write balanced chemical equation for the reaction between Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Molarity of naoh = (mol khp)/ (v naoh used for titration): To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED A student titrated 1.0115 g sample of potassium hydrogen Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. Report the determined concentration of the. Molarity of naoh = (mol khp)/ (v naoh used for titration): One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. To standardize a sodium hydroxide. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved 1. Write the balanced chemical reaction between Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Report the determined concentration of the. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Potassium hydrogen phthalate and sodium hydroxide react as follows: Molarity of naoh = (mol khp)/. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From studylib.net
Titration of the Weak Acid Potassium Hydrogen Phthalate Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. To standardize a solution of sodium hydroxide by titration with a primary standard, potassium hydrogen phthalate. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Standardize a sodium hydroxide (naoh) solution using titration of potassium. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.chegg.com
Solved A pH titration of a potassium hydrogen phthalate Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation One such compound is potassium hydrogen phthalate (khp), a weak monoprotic acid suitable for standardizing solutions of bases such as sodium hydroxide. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED Terms used in Titration Please match the following responses Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a 0.1421 g sample of potassium hydrogen phthalate (khp). Khc8h4o4 + naoh →. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.
From www.numerade.com
SOLVED Write a balanced equation for the neutralization of potassium Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation Molarity of naoh = (mol khp)/ (v naoh used for titration): Standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Khc8h4o4 + naoh → knac8h4o4 + h2o or, expressed as an ionic. Report the determined concentration of the. An aqueous solution of sodium hydroxide (naoh) was standardized by titrating it against a. Titration Of Potassium Hydrogen Phthalate With Sodium Hydroxide Equation.