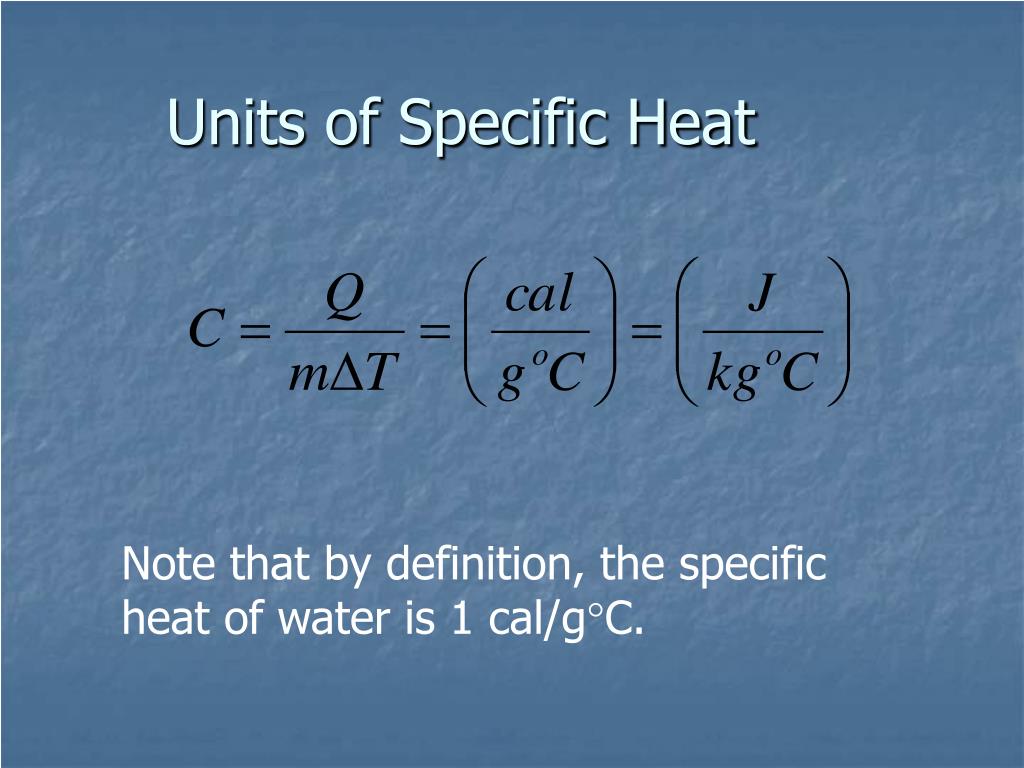
Heat Unit Definition Science . Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. If two bodies at different. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. How is it generated & transferred. What are units of heat? Learn its meaning, facts, types, formula, & symbol, along with images. What is thermal (heat) energy. Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is what scientists call the form of energy that is transferred between two materials of different temperature.
from www.slideserve.com
Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. What are units of heat? Heat is what scientists call the form of energy that is transferred between two materials of different temperature. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Learn its meaning, facts, types, formula, & symbol, along with images. Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. What is thermal (heat) energy. If two bodies at different. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference.
PPT Thermodynamics PowerPoint Presentation, free download ID1596982
Heat Unit Definition Science Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. Heat, energy that is transferred from one body to another as the result of a difference in temperature. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. What is thermal (heat) energy. If two bodies at different. What are units of heat? Heat is what scientists call the form of energy that is transferred between two materials of different temperature. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Learn its meaning, facts, types, formula, & symbol, along with images. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. How is it generated & transferred.
From gamesmartz.com
Heat Easy to Understand Definition Heat Unit Definition Science Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. What are units of heat?. Heat Unit Definition Science.
From www.dreamstime.com
Heat Energy As Convection, Conduction and Radiation, Physics Science Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Learn its meaning, facts, types, formula, & symbol, along with images. Heat is the amount of energy. Heat Unit Definition Science.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Unit Definition Science Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Learn its meaning, facts, types, formula, & symbol, along with images. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. What is thermal (heat) energy. Heat, energy that is transferred from one body to another as. Heat Unit Definition Science.
From www.sliderbase.com
Thermodynamics Presentation Chemistry Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications.. Heat Unit Definition Science.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Heat Unit Definition Science Heat is the thermal energy transfer between systems or bodies due to a temperature difference. How is it generated & transferred. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. What is thermal (heat) energy. Heat, as a form of energy, is quantified using several units that are essential in various. Heat Unit Definition Science.
From gamesmartz.com
Heat Capacity Definition Easy to Understand Heat Unit Definition Science How is it generated & transferred. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. What is thermal (heat) energy. Thermal energy, in turn, is the kinetic energy of. Heat Unit Definition Science.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Unit Definition Science What are units of heat? What is thermal (heat) energy. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Learn its meaning, facts, types, formula, & symbol, along with images. Heat is the. Heat Unit Definition Science.
From becuo.com
Heat Transfer Images & Pictures Becuo Heat Unit Definition Science What are units of heat? What is thermal (heat) energy. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. How is it generated & transferred. Heat,. Heat Unit Definition Science.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID1596982 Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat, as a form of energy, is quantified using several units that are essential in various scientific. Heat Unit Definition Science.
From www.britannica.com
Specific heat physics Britannica Heat Unit Definition Science Heat is what scientists call the form of energy that is transferred between two materials of different temperature. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Learn its meaning, facts, types,. Heat Unit Definition Science.
From www.youtube.com
Modes of heat transfer UNIT MATTER(Part4) Grade 7,8 Science Heat Unit Definition Science What are units of heat? Heat is what scientists call the form of energy that is transferred between two materials of different temperature. If two bodies at different. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Learn its meaning, facts, types, formula, & symbol, along with images. Thermal energy, in turn, is the. Heat Unit Definition Science.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. If two bodies at different. Learn its meaning, facts, types, formula, & symbol, along with. Heat Unit Definition Science.
From www.slideshare.net
Heat Capacity Heat Unit Definition Science Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. Heat, energy that is transferred from one body to another as the result of a difference in temperature. What are units of heat? Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat is the amount of. Heat Unit Definition Science.
From www.sliderbase.com
Heat Presentation Chemistry Heat Unit Definition Science Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. What is thermal (heat) energy. How is it generated & transferred. Heat is what scientists. Heat Unit Definition Science.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Heat Unit Definition Science Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat, as a form of energy, is quantified using several units that are essential in. Heat Unit Definition Science.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Heat Unit Definition Science Thermal energy, in turn, is the kinetic energy of vibrating and colliding. What is thermal (heat) energy. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. Learn its meaning, facts, types,. Heat Unit Definition Science.
From www.mechanicaleducation.com
Difference between Heat and Temperature Mechanical Education Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. What are units of heat? How is it generated & transferred. Heat is the thermal. Heat Unit Definition Science.
From sciencenotes.org
What Is Temperature? Definition in Science Heat Unit Definition Science Thermal energy, in turn, is the kinetic energy of vibrating and colliding. How is it generated & transferred. Heat, energy that is transferred from one body to another as the result of a difference in temperature. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific. Heat Unit Definition Science.
From www.slideserve.com
PPT Thermal Physics IB Physics PowerPoint Presentation, free download Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference.. Heat Unit Definition Science.
From www.youtube.com
Unit of heat energy SI unit of heat CGS unit of heat Joule Heat Unit Definition Science Heat, energy that is transferred from one body to another as the result of a difference in temperature. What is thermal (heat) energy. What are units of heat? Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. If two bodies at different. How is it generated & transferred. Heat. Heat Unit Definition Science.
From www.flinnsci.com
360 Science Thermal Energy and Heat Transfer Heat Unit Definition Science Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. If two bodies at different. What is thermal (heat) energy. How is it generated & transferred. What are units of heat? Learn its meaning, facts, types, formula, & symbol, along with images. Heat is what scientists call the form of. Heat Unit Definition Science.
From education-portal.com
Latent Heat Definition, Formula & Examples Video & Lesson Transcript Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Learn its meaning, facts, types, formula, & symbol, along with images. What is thermal (heat) energy. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. If two bodies at different. Heat is. Heat Unit Definition Science.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint Heat Unit Definition Science Heat, energy that is transferred from one body to another as the result of a difference in temperature. If two bodies at different. What are units of heat? Learn its meaning, facts, types, formula, & symbol, along with images. What is thermal (heat) energy. How is it generated & transferred. Heat is the thermal energy transfer between systems or bodies. Heat Unit Definition Science.
From www.saralmind.com
Heat Notes, Videos, QA and Tests Class 10>Science>Heat SaralMind Heat Unit Definition Science How is it generated & transferred. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat is the amount of energy that is transferred. Heat Unit Definition Science.
From www.slideserve.com
PPT Specific Heat vs. Heat capacity PowerPoint Presentation, free Heat Unit Definition Science What are units of heat? Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. Heat, energy that is transferred from one body to another as the result of a difference in temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat, as. Heat Unit Definition Science.
From www.youtube.com
Specific heat capacity YouTube Heat Unit Definition Science Learn its meaning, facts, types, formula, & symbol, along with images. If two bodies at different. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. How is it generated & transferred. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. What are units of heat? The joule (j),. Heat Unit Definition Science.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID831852 Heat Unit Definition Science What is thermal (heat) energy. Heat, energy that is transferred from one body to another as the result of a difference in temperature. What are units of heat? Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat is what scientists call the form of energy that is transferred between two materials of different. Heat Unit Definition Science.
From www.pinterest.com
Specific Heat Easy Science Teaching chemistry, Sensible heat Heat Unit Definition Science Heat, energy that is transferred from one body to another as the result of a difference in temperature. Learn its meaning, facts, types, formula, & symbol, along with images. If two bodies at different. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Thermal. Heat Unit Definition Science.
From ar.inspiredpencil.com
Heat Science Definition Heat Unit Definition Science How is it generated & transferred. What is thermal (heat) energy. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. Heat, as a form of energy,. Heat Unit Definition Science.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID2649066 Heat Unit Definition Science Heat, as a form of energy, is quantified using several units that are essential in various scientific and practical applications. Learn its meaning, facts, types, formula, & symbol, along with images. How is it generated & transferred. Heat, energy that is transferred from one body to another as the result of a difference in temperature. What are units of heat?. Heat Unit Definition Science.
From animalia-life.club
Examples Of Heat Energy Heat Unit Definition Science Heat is what scientists call the form of energy that is transferred between two materials of different temperature. How is it generated & transferred. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as. Heat Unit Definition Science.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Unit Definition Science What is thermal (heat) energy. Heat is what scientists call the form of energy that is transferred between two materials of different temperature. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat. Heat Unit Definition Science.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free Heat Unit Definition Science Heat is what scientists call the form of energy that is transferred between two materials of different temperature. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is the amount of energy that is transferred from one system to its surroundings because of. Heat Unit Definition Science.
From www.sliderbase.com
Thermal Energy Presentation Physics Heat Unit Definition Science The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. Heat is what scientists call the form of energy that is transferred between two materials. Heat Unit Definition Science.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID831852 Heat Unit Definition Science Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat is the amount of energy that is transferred from one system to its surroundings because of a temperature difference. The joule (j), the si unit for energy, is also used to measure heat, reflecting the amount of energy transferred as heat in scientific contexts. Learn its meaning,. Heat Unit Definition Science.