Titration Experiment Of Naoh And Hcl . An indicator that changes color when the ph becomes greater than 7 (more. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. In this experiment, a strong base (naoh) is being added to a strong acid (hcl). Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The initial volume and final volume of all three trails were subtracted to find each amount of. Volume measurements play a key role in titration. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Back titration of hcl with standard naoh. Hcl + naoh → nacl + h 2 o.
from www.chemistryscl.com
The initial volume and final volume of all three trails were subtracted to find each amount of. An indicator that changes color when the ph becomes greater than 7 (more. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volume measurements play a key role in titration. Hcl + naoh → nacl + h 2 o. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. In this experiment, a strong base (naoh) is being added to a strong acid (hcl).
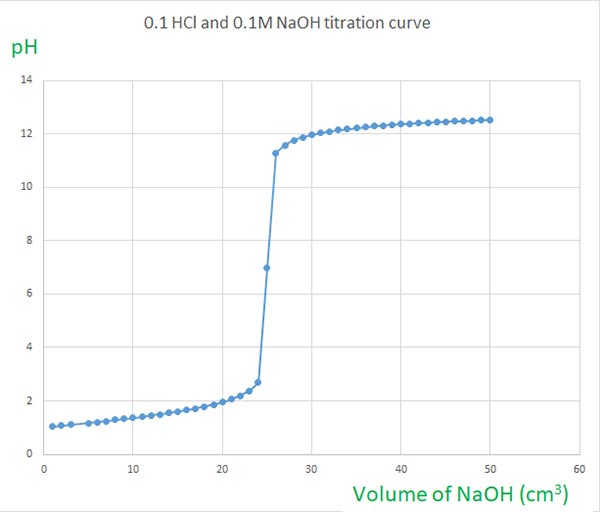
NaOH and HCl Titration Curves Selecting Indicators
Titration Experiment Of Naoh And Hcl Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Back titration of hcl with standard naoh. Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Hcl + naoh → nacl + h 2 o. In this experiment, a strong base (naoh) is being added to a strong acid (hcl). The initial volume and final volume of all three trails were subtracted to find each amount of. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volume measurements play a key role in titration. Includes kit list and safety instructions. An indicator that changes color when the ph becomes greater than 7 (more.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Experiment Of Naoh And Hcl Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Hcl + naoh → nacl + h 2 o. Back titration of hcl with standard naoh. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Both acid and base are strong, which not only makes determination. Titration Experiment Of Naoh And Hcl.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Experiment Of Naoh And Hcl Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In this experiment, a strong base (naoh) is being added to a strong acid (hcl). Includes kit list and safety instructions. The initial volume and final volume of all three trails were subtracted to find each amount of. A titration is an. Titration Experiment Of Naoh And Hcl.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Experiment Of Naoh And Hcl In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Volume measurements play a key role in titration. Back titration of hcl with standard naoh. Includes kit list and safety instructions. Hcl + naoh → nacl + h 2 o. Both acid and base are strong, which not only makes determination of. Titration Experiment Of Naoh And Hcl.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Experiment Of Naoh And Hcl The initial volume and final volume of all three trails were subtracted to find each amount of. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Hcl + naoh → nacl + h 2 o. An indicator that changes color when the. Titration Experiment Of Naoh And Hcl.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Experiment Of Naoh And Hcl Volume measurements play a key role in titration. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. An indicator that changes color when the ph becomes greater than 7 (more. A titration is an experiment where a volume of a solution. Titration Experiment Of Naoh And Hcl.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Experiment Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The initial volume and final volume of all three trails were subtracted to find each amount of. Includes kit list and safety instructions. Both acid and base are strong, which not only. Titration Experiment Of Naoh And Hcl.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Experiment Of Naoh And Hcl A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment, a strong base (naoh) is being added to a strong acid (hcl). An indicator that changes color when the ph becomes greater than 7 (more. In the neutralization of hydrochloric acid by. Titration Experiment Of Naoh And Hcl.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Of Naoh And Hcl Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Includes kit list and safety instructions. The initial volume and final volume of all three trails were subtracted to find each amount of. In this experiment, a strong. Titration Experiment Of Naoh And Hcl.
From www.studocu.com
Experiment 3 (Manual) Experiment 3 Titration analysis, Titration of Titration Experiment Of Naoh And Hcl Back titration of hcl with standard naoh. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Volume measurements play a key role in titration. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. A titration is an experiment where a volume of a. Titration Experiment Of Naoh And Hcl.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Experiment Of Naoh And Hcl In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Includes kit list and safety instructions. Back titration of hcl with standard naoh. The initial volume and final volume of all three trails were subtracted to find each amount of. Volume measurements play a key role in titration. An indicator that changes. Titration Experiment Of Naoh And Hcl.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Experiment Of Naoh And Hcl In this experiment, a strong base (naoh) is being added to a strong acid (hcl). Back titration of hcl with standard naoh. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Use this class practical to explore titration, producing the salt. Titration Experiment Of Naoh And Hcl.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Titration Experiment Of Naoh And Hcl Volume measurements play a key role in titration. The initial volume and final volume of all three trails were subtracted to find each amount of. Back titration of hcl with standard naoh. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Hydrochloric acid reacts. Titration Experiment Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Experiment Of Naoh And Hcl In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volume measurements play a key role in titration. The initial volume and final volume of all three. Titration Experiment Of Naoh And Hcl.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titration Experiment Of Naoh And Hcl Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. An indicator that changes color when the ph becomes greater than 7 (more. Volume measurements play a key role in titration. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to. Titration Experiment Of Naoh And Hcl.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Titration Experiment Of Naoh And Hcl Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. The initial volume and final volume of all three trails were. Titration Experiment Of Naoh And Hcl.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Titration Experiment Of Naoh And Hcl Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. The initial volume and final volume of all three trails were subtracted to find each amount of. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In. Titration Experiment Of Naoh And Hcl.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Titration Experiment Of Naoh And Hcl An indicator that changes color when the ph becomes greater than 7 (more. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. The initial volume and final volume of all three trails were. Titration Experiment Of Naoh And Hcl.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Experiment Of Naoh And Hcl Hcl + naoh → nacl + h 2 o. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In this experiment, a strong base (naoh) is being. Titration Experiment Of Naoh And Hcl.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Experiment Of Naoh And Hcl The initial volume and final volume of all three trails were subtracted to find each amount of. An indicator that changes color when the ph becomes greater than 7 (more. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Hcl + naoh → nacl + h 2 o. Both acid and base. Titration Experiment Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Experiment Of Naoh And Hcl Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Back titration of hcl with standard naoh. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. In the neutralization of. Titration Experiment Of Naoh And Hcl.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titration Experiment Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. In this experiment, a strong base (naoh) is being added to a strong acid (hcl). Includes kit list and safety instructions. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Use this class practical to explore titration, producing the salt sodium. Titration Experiment Of Naoh And Hcl.
From studylib.net
Titration of HCl with NaOH Titration Experiment Of Naoh And Hcl Volume measurements play a key role in titration. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. In this. Titration Experiment Of Naoh And Hcl.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Experiment Of Naoh And Hcl Back titration of hcl with standard naoh. Hcl + naoh → nacl + h 2 o. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Use this. Titration Experiment Of Naoh And Hcl.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Experiment Of Naoh And Hcl In this experiment, a strong base (naoh) is being added to a strong acid (hcl). An indicator that changes color when the ph becomes greater than 7 (more. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Back titration of hcl with standard naoh. In the neutralization of hydrochloric acid by. Titration Experiment Of Naoh And Hcl.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Experiment Of Naoh And Hcl Back titration of hcl with standard naoh. An indicator that changes color when the ph becomes greater than 7 (more. The initial volume and final volume of all three trails were subtracted to find each amount of. Volume measurements play a key role in titration. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Hcl + naoh → nacl. Titration Experiment Of Naoh And Hcl.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Experiment Of Naoh And Hcl Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. The initial volume and. Titration Experiment Of Naoh And Hcl.
From www.science-revision.co.uk
Titrations Titration Experiment Of Naoh And Hcl Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Hcl + naoh → nacl + h 2 o. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. In the neutralization of hydrochloric acid. Titration Experiment Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Experiment Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. An indicator that changes color when the ph becomes greater than 7 (more. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Hcl + naoh → nacl + h 2 o. A titration is an experiment where a volume of a. Titration Experiment Of Naoh And Hcl.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Experiment Of Naoh And Hcl Back titration of hcl with standard naoh. Volume measurements play a key role in titration. Includes kit list and safety instructions. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. Hydrochloric acid reacts with. Titration Experiment Of Naoh And Hcl.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Experiment Of Naoh And Hcl Both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of titration curve and equivalence. Volume measurements play a key role in titration. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. The initial. Titration Experiment Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Titration Experiment Of Naoh And Hcl An indicator that changes color when the ph becomes greater than 7 (more. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Using. Titration Experiment Of Naoh And Hcl.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Experiment Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. Back titration of hcl with standard naoh. Volume measurements play a key role in titration. In this experiment, a strong base (naoh) is being added to a strong acid (hcl). A titration is an experiment where a volume of a solution of known concentration. Titration Experiment Of Naoh And Hcl.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Experiment Of Naoh And Hcl Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. An indicator that changes color when the ph becomes greater than 7 (more. The initial volume and. Titration Experiment Of Naoh And Hcl.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Experiment Of Naoh And Hcl In this experiment, a strong base (naoh) is being added to a strong acid (hcl). An indicator that changes color when the ph becomes greater than 7 (more. Hcl + naoh → nacl + h 2 o. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in. Titration Experiment Of Naoh And Hcl.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Experiment Of Naoh And Hcl An indicator that changes color when the ph becomes greater than 7 (more. Using the analytical balance, weigh out three samples (nominally about 0.13 g, but weighed to 0.1 mg. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Volume measurements play a key. Titration Experiment Of Naoh And Hcl.