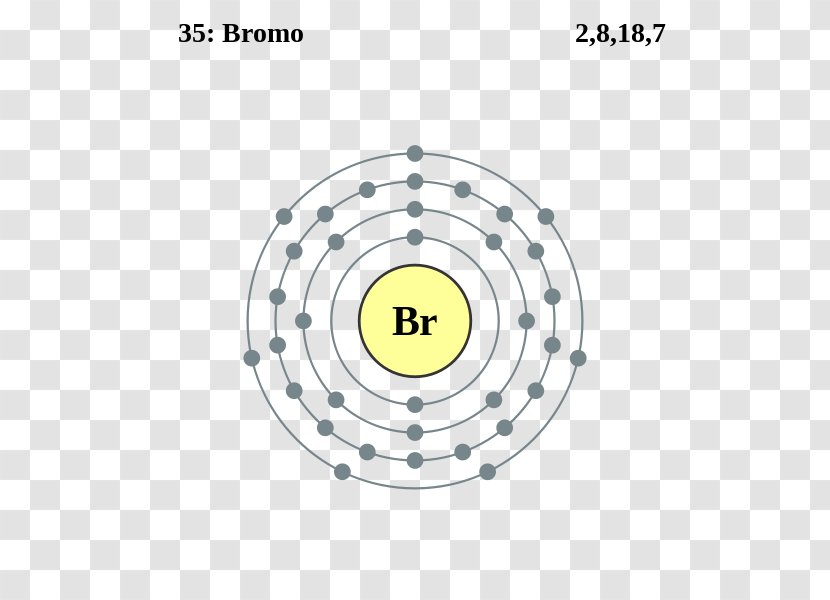
Bromine Electron Withdrawing . The bromine substitution of nitrobenzene. Groups that can donate electron density to the. For electron withdrawing groups, all of. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. does the substituent activate or deactivate the aromatic ring? the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This is attacked by the.
from fyoyriozh.blob.core.windows.net
Groups that can donate electron density to the. does the substituent activate or deactivate the aromatic ring? This is attacked by the. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Where will the incoming group go? The bromine substitution of nitrobenzene. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of.
Bromine Electron Configuration Long at Philip Harbin blog
Bromine Electron Withdrawing The bromine substitution of nitrobenzene. This is attacked by the. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Groups that can donate electron density to the. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Where will the incoming group go? For electron withdrawing groups, all of. The bromine substitution of nitrobenzene. does the substituent activate or deactivate the aromatic ring? the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Withdrawing This is attacked by the. For electron withdrawing groups, all of. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Groups that can donate electron density to. Bromine Electron Withdrawing.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromine Electron Withdrawing Where will the incoming group go? The bromine substitution of nitrobenzene. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. For electron withdrawing groups, all of. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. Groups. Bromine Electron Withdrawing.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network Bromine Electron Withdrawing Where will the incoming group go? as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. The bromine substitution of nitrobenzene. Groups that can donate electron density to the. This is. Bromine Electron Withdrawing.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Electron Withdrawing Groups that can donate electron density to the. This is attacked by the. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. Where will the incoming group. Bromine Electron Withdrawing.
From exyvbfgyn.blob.core.windows.net
Fluorine Bromine Electron Configuration at Tracey Engle blog Bromine Electron Withdrawing The bromine substitution of nitrobenzene. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Groups that can donate electron density to the. Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. the. Bromine Electron Withdrawing.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Bromine Electron Withdrawing does the substituent activate or deactivate the aromatic ring? Groups that can donate electron density to the. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity.. Bromine Electron Withdrawing.
From fyoyriozh.blob.core.windows.net
Bromine Electron Configuration Long at Philip Harbin blog Bromine Electron Withdrawing the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. This is attacked. Bromine Electron Withdrawing.
From www.alamy.com
Bromine molecular model Stock Vector Images Alamy Bromine Electron Withdrawing Groups that can donate electron density to the. For electron withdrawing groups, all of. Where will the incoming group go? This is attacked by the. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. The bromine substitution of nitrobenzene. does the substituent activate or deactivate the aromatic ring? as. Bromine Electron Withdrawing.
From www.chegg.com
Solved Aldehydes and ketones can be halogenated at their Bromine Electron Withdrawing The bromine substitution of nitrobenzene. This is attacked by the. Groups that can donate electron density to the. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. For electron withdrawing groups, all of. Where will the. Bromine Electron Withdrawing.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Withdrawing as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. does the substituent activate or deactivate the aromatic ring? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Groups that can donate electron density to the. This is attacked. Bromine Electron Withdrawing.
From pubs.acs.org
Impact of ElectronWithdrawing Effect on Sigma Hole Via BromineBased Bromine Electron Withdrawing This is attacked by the. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Groups that can donate electron density to the. does the substituent activate. Bromine Electron Withdrawing.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Electron Withdrawing Where will the incoming group go? does the substituent activate or deactivate the aromatic ring? The bromine substitution of nitrobenzene. For electron withdrawing groups, all of. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. the stepwise bromination of an alkene involves the. Bromine Electron Withdrawing.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Withdrawing does the substituent activate or deactivate the aromatic ring? This is attacked by the. For electron withdrawing groups, all of. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Groups that can donate electron density to the. Where will the incoming group go? The. Bromine Electron Withdrawing.
From www.alamy.com
Bromine Chemical Element Graphic for Science Designs Stock Vector Image Bromine Electron Withdrawing The bromine substitution of nitrobenzene. Where will the incoming group go? Groups that can donate electron density to the. For electron withdrawing groups, all of. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. does the substituent activate or deactivate the aromatic ring? as we saw in section 2.1, an inductive effect. Bromine Electron Withdrawing.
From www.vrogue.co
What Is The Meaning Of Cis Trans Ortho Meta And Para vrogue.co Bromine Electron Withdrawing For electron withdrawing groups, all of. Groups that can donate electron density to the. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. does the substituent activate or deactivate. Bromine Electron Withdrawing.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Electron Withdrawing Groups that can donate electron density to the. The bromine substitution of nitrobenzene. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. For electron withdrawing groups, all. Bromine Electron Withdrawing.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Electron Withdrawing Where will the incoming group go? Groups that can donate electron density to the. This is attacked by the. does the substituent activate or deactivate the aromatic ring? For electron withdrawing groups, all of. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. electron withdrawing groups destabilize the sigma. Bromine Electron Withdrawing.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Withdrawing the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. This is attacked by the. The bromine substitution of nitrobenzene. For electron withdrawing groups, all of. does the substituent activate or deactivate the aromatic ring? as we saw in section 2.1, an inductive effect is the withdrawal or donation of. Bromine Electron Withdrawing.
From giofwcqdg.blob.core.windows.net
Chemical Formula Bromine Lithium at Jeffrey Heinz blog Bromine Electron Withdrawing electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of. Where will the incoming group go? The bromine substitution of nitrobenzene. does the substituent activate or deactivate the aromatic ring? as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through. Bromine Electron Withdrawing.
From fyoekyjrv.blob.core.windows.net
Electron Configuration Of Bromine Long Form at Lowell Sadowski blog Bromine Electron Withdrawing Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This is attacked by the. Groups that can donate electron density to the. does the substituent activate or deactivate the aromatic ring? For electron withdrawing groups, all of. The bromine substitution of nitrobenzene. the stepwise bromination of. Bromine Electron Withdrawing.
From fyoekyjrv.blob.core.windows.net
Electron Configuration Of Bromine Long Form at Lowell Sadowski blog Bromine Electron Withdrawing For electron withdrawing groups, all of. Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. does the substituent activate or deactivate the aromatic ring? Groups that can donate electron density to the. as we saw in section 2.1, an inductive effect is the withdrawal or donation. Bromine Electron Withdrawing.
From xkldase.edu.vn
Share more than 113 ring activating and deactivating groups best Bromine Electron Withdrawing does the substituent activate or deactivate the aromatic ring? as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Groups that can donate electron density to the. Where will the incoming group go? For electron withdrawing groups, all of. The bromine substitution of nitrobenzene. This. Bromine Electron Withdrawing.
From www.masterorganicchemistry.com
EAS On Disubstituted Benzenes The Strongest ElectronDonor "Wins" Bromine Electron Withdrawing electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. The bromine substitution of nitrobenzene. Groups that can donate electron density to the. does the substituent activate or deactivate the aromatic ring? This is attacked by the. For electron withdrawing groups, all of. as we saw in section 2.1, an inductive effect is. Bromine Electron Withdrawing.
From www.numerade.com
SOLVED Aldehydes and ketones can be halogenated at their position by Bromine Electron Withdrawing The bromine substitution of nitrobenzene. Groups that can donate electron density to the. For electron withdrawing groups, all of. does the substituent activate or deactivate the aromatic ring? as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. the stepwise bromination of an alkene. Bromine Electron Withdrawing.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity Bromine Electron Withdrawing Where will the incoming group go? This is attacked by the. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. The bromine substitution of nitrobenzene. Groups that can donate electron density to the. does the substituent activate or deactivate the aromatic ring? For electron withdrawing groups, all of. electron. Bromine Electron Withdrawing.
From games.assurances.gov.gh
Bromine Electron Configuration Long Form Bromine Electron Withdrawing Where will the incoming group go? For electron withdrawing groups, all of. This is attacked by the. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. The bromine substitution of nitrobenzene. does the substituent activate. Bromine Electron Withdrawing.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Withdrawing For electron withdrawing groups, all of. This is attacked by the. The bromine substitution of nitrobenzene. Where will the incoming group go? the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. as we saw in. Bromine Electron Withdrawing.
From app.emaze.com
Bromine ) on emaze Bromine Electron Withdrawing Groups that can donate electron density to the. The bromine substitution of nitrobenzene. For electron withdrawing groups, all of. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. Where will the incoming group go? This is attacked by the. the stepwise bromination of an. Bromine Electron Withdrawing.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Electron Withdrawing The bromine substitution of nitrobenzene. Groups that can donate electron density to the. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Where will the incoming group go? the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. For electron withdrawing groups, all of. This is. Bromine Electron Withdrawing.
From proper-cooking.info
Bromine Orbital Diagram Bromine Electron Withdrawing does the substituent activate or deactivate the aromatic ring? This is attacked by the. For electron withdrawing groups, all of. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give. Bromine Electron Withdrawing.
From techiescientist.com
Is Br2 Polar or Nonpolar? Techiescientist Bromine Electron Withdrawing For electron withdrawing groups, all of. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. The bromine substitution of nitrobenzene. This is attacked by the. does the substituent activate. Bromine Electron Withdrawing.
From chem.ucalgary.ca
Chapter 24 Phenols Bromine Electron Withdrawing Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. The bromine substitution of nitrobenzene. For electron withdrawing groups, all of. the stepwise bromination. Bromine Electron Withdrawing.
From www.pearson.com
Electron Withdrawing Groups Online Tutor, Practice Problems & Exam Prep Bromine Electron Withdrawing This is attacked by the. The bromine substitution of nitrobenzene. does the substituent activate or deactivate the aromatic ring? For electron withdrawing groups, all of. the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. as we saw in section 2.1, an inductive effect is the withdrawal or donation of. Bromine Electron Withdrawing.
From www.researchgate.net
Design strategies for developing cell permeable fluorophores. (a Bromine Electron Withdrawing the stepwise bromination of an alkene involves the nucleophilic alkene attacking bromine to give a bromonium ion. The bromine substitution of nitrobenzene. as we saw in section 2.1, an inductive effect is the withdrawal or donation of electrons through a σ bond due to electronegativity. This is attacked by the. Where will the incoming group go? electron. Bromine Electron Withdrawing.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution Introduction and Mechanism Bromine Electron Withdrawing Where will the incoming group go? electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. The bromine substitution of nitrobenzene. Groups that can donate electron density to the. For electron withdrawing groups, all of. does the substituent activate or deactivate the aromatic ring? This is attacked by the. the stepwise bromination of. Bromine Electron Withdrawing.