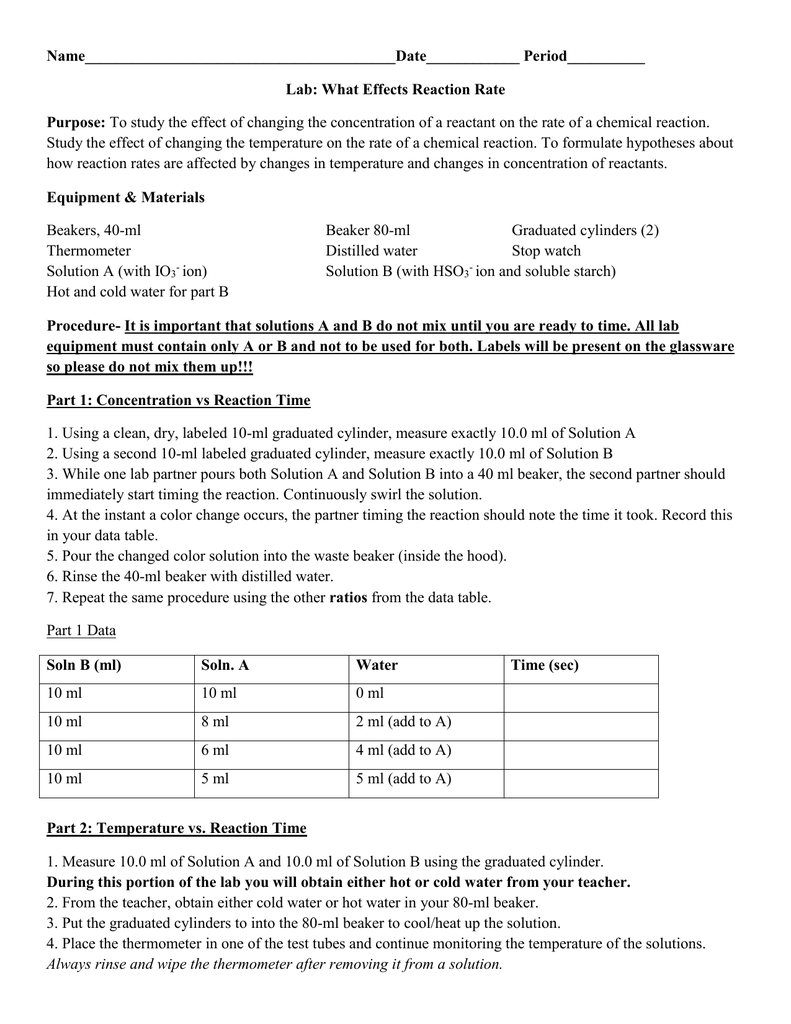
Lab Reaction Rate . The reaction rate would be slower than the rate at 95°c. The rate of reaction is the change in the amount of a reactant or product per unit time. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. Did the amount of catalyst affect the reaction rate? What follows is general guidance and. Reaction rates are therefore determined by measuring the time. Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. The rate of reaction is the change in the amount of a reactant or product per unit time. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. Study with quizlet and memorize. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. During this experiment we actively tried to answer the question; The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The reaction rate would be equal to the rate at 65°c. Reaction rates are therefore determined by.
from studylib.net
The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by. Reaction rates are therefore determined by measuring the time. What follows is general guidance and. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The reaction rate would be equal to the rate at 65°c. The rate of reaction is the change in the amount of a reactant or product per unit time. Did the amount of catalyst affect the reaction rate? Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. During this experiment we actively tried to answer the question;
Lab Reaction Rates
Lab Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. The rate of reaction is the change in the amount of a reactant or product per unit time. The reaction rate would be equal to the rate at 65°c. The rate of reaction is the change in the amount of a reactant or product per unit time. During this experiment we actively tried to answer the question; Did the amount of catalyst affect the reaction rate? Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. Reaction rates are therefore determined by measuring the time. What follows is general guidance and. The reaction rate would be slower than the rate at 95°c. Study with quizlet and memorize. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. Reaction rates are therefore determined by.
From www.chegg.com
Solved REPORT SHEET LAB 18 Reaction Rates and Chemical Lab Reaction Rate Reaction rates are therefore determined by measuring the time. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. Did the amount of catalyst affect the reaction rate? During this experiment we actively tried to answer the question; Reaction rates are therefore determined by. The reaction rate would. Lab Reaction Rate.
From studylib.net
Lab 27 The Effect of Concentration on Reaction Rate Purpose Lab Reaction Rate Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. What follows is general guidance and. Did the amount of. Lab Reaction Rate.
From www.studypool.com
SOLUTION Chemistry b unit 1 lab reaction rate student guide Studypool Lab Reaction Rate Reaction rates are therefore determined by. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. The reaction rate would be equal to the rate at 65°c. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence. Lab Reaction Rate.
From www.chegg.com
Solved Lab 3 Reaction Rates Data Table 1. Nature of the Lab Reaction Rate During this experiment we actively tried to answer the question; In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The rate of reaction is the change in. Lab Reaction Rate.
From www.scribd.com
Rates of Reaction Experiment v.1.02 Reaction Rate Chemical Reactions Lab Reaction Rate Study with quizlet and memorize. What follows is general guidance and. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The reaction rate would be slower than the rate at 95°c. The rate of reaction is the change in the amount of a reactant or product per. Lab Reaction Rate.
From www.youtube.com
Lab Experiment 19 Effect of Concentration on the Reaction Rate. YouTube Lab Reaction Rate Study with quizlet and memorize. During this experiment we actively tried to answer the question; What follows is general guidance and. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. Reaction rates are therefore determined by. The reaction rate would be equal to the rate at 65°c. Reaction rates. Lab Reaction Rate.
From www.studypool.com
SOLUTION Chemistry b unit 1 lab reaction rate student guide Studypool Lab Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. Did the amount of catalyst affect the reaction rate? The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. Study with quizlet and memorize. In this lab we explored the. Lab Reaction Rate.
From www.youtube.com
How to do lab report [Exp 004] Rates of Reaction for Iodine Clock Lab Reaction Rate The reaction rate would be slower than the rate at 95°c. Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. What follows is general guidance and. During this experiment we actively tried to answer the question; Reaction rates are therefore determined by. Reaction rates are therefore determined. Lab Reaction Rate.
From www.youtube.com
Reaction Rate Lab Chemistry Matters YouTube Lab Reaction Rate Reaction rates are therefore determined by. What follows is general guidance and. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The rate of reaction is the change in the amount of a reactant or product per unit time. The reaction rate would be slower than the. Lab Reaction Rate.
From studylib.net
Reaction rate lab Lab Reaction Rate During this experiment we actively tried to answer the question; The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time. What follows is general guidance and. The rate of reaction is the change in the amount of a reactant or product per unit. Lab Reaction Rate.
From myriverside.sd43.bc.ca
Graph Factors Affecting Reaction Rate lab Lauren's Blog Lab Reaction Rate The reaction rate would be equal to the rate at 65°c. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. During this experiment we actively tried to answer the question; In this lab we explored the rate of a chemical reaction at different temperatures and with different. Lab Reaction Rate.
From studylib.net
The effect of temperature on reaction rate lab Lab Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. Study with quizlet and memorize. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Reaction rates are therefore determined by. The rate of reaction is the change in the amount of a reactant or product. Lab Reaction Rate.
From www.scribd.com
lab report reaction rate Hydrochloric Acid Experiment Lab Reaction Rate The reaction rate would be slower than the rate at 95°c. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Reaction rates are therefore determined by. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. What follows is general guidance. Lab Reaction Rate.
From studylib.net
Lab Reaction Rates Lab Reaction Rate Study with quizlet and memorize. Reaction rates are therefore determined by. The rate of reaction is the change in the amount of a reactant or product per unit time. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The rate of reaction is the change in the. Lab Reaction Rate.
From www.scribd.com
Lab Reaction Rate Lab PDF Lab Reaction Rate What follows is general guidance and. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Did the amount of catalyst affect the reaction rate? The rate of reaction is the change. Lab Reaction Rate.
From studylib.net
Rates of Reaction Lab Report Lab Reaction Rate Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of. The reaction rate would be slower than the rate at 95°c. During this experiment we actively. Lab Reaction Rate.
From about.dataclassroom.com
Reaction Rate Lab — DataClassroom Lab Reaction Rate In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. Study with quizlet and memorize. The reaction rate would be slower than the rate at 95°c. The reaction rate would be equal to the rate at 65°c. Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate. Lab Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 Lab Reaction Rate What follows is general guidance and. The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. During this experiment we actively tried to. Lab Reaction Rate.
From studylib.net
Factors Affecting Reaction Rate Lab. Lab Reaction Rate Reaction rates are therefore determined by. The rate of reaction is the change in the amount of a reactant or product per unit time. The reaction rate would be slower than the rate at 95°c. Did the amount of catalyst affect the reaction rate? Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction. Lab Reaction Rate.
From www.youtube.com
IGCSE Chemistry Rates of Reaction YouTube Lab Reaction Rate Did the amount of catalyst affect the reaction rate? In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. During this experiment we actively tried to answer the question; The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration. Lab Reaction Rate.
From www.studocu.com
Lab Report Reaction Rate Lab During this experiment we actively tried Lab Reaction Rate What follows is general guidance and. The rate of reaction is the change in the amount of a reactant or product per unit time. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. The reaction rate would be slower than the rate at 95°c. Did the amount of catalyst. Lab Reaction Rate.
From www.studocu.com
chemistry lab report Lab Reaction Rate Purpose To see how different Lab Reaction Rate Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. The rate of reaction is the change in the amount of a reactant or product per unit time. What follows is general guidance and. With the obtained data, it is possible to. Lab Reaction Rate.
From mathequalslove.net
Effect of Concentration on Reaction Rate Lab Math = Love Lab Reaction Rate Reaction rates are therefore determined by. The rate of reaction is the change in the amount of a reactant or product per unit time. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. What follows is general guidance and. In this lab we explored the rate of a chemical reaction at different temperatures. Lab Reaction Rate.
From studylib.net
Notes Unit 74 Lab Reaction rate Lab Reaction Rate Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. The reaction rate would be equal to the rate at 65°c. Reaction rates are therefore determined by measuring the time. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant. Lab Reaction Rate.
From dxohbgnkv.blob.core.windows.net
Rate Of Reaction Lab Experiment at Gerald Steele blog Lab Reaction Rate In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Reaction rates are therefore determined by measuring the time. The rate of reaction is the change in the amount of a reactant or product. Lab Reaction Rate.
From www.scribd.com
Lab 14_Effects of Temperature on Reaction Rate Lab Reaction Rate During this experiment we actively tried to answer the question; Did the amount of catalyst affect the reaction rate? The rate of reaction is the change in the amount of a reactant or product per unit time. What follows is general guidance and. The rate of reaction is the change in the amount of a reactant or product per unit. Lab Reaction Rate.
From dxohbgnkv.blob.core.windows.net
Rate Of Reaction Lab Experiment at Gerald Steele blog Lab Reaction Rate What follows is general guidance and. Did the amount of catalyst affect the reaction rate? Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. The reaction rate would be equal to the rate at 65°c. With the obtained data, it is possible to calculate the reaction rate. Lab Reaction Rate.
From studylib.net
Lab Reaction Rate Lab Reaction Rate The reaction rate would be equal to the rate at 65°c. Study with quizlet and memorize. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Reaction rates are therefore determined by. Did the amount of catalyst affect the reaction rate? The rate law of a chemical reaction is a mathematical equation that describes. Lab Reaction Rate.
From www.coursehero.com
[Solved] Experiment 1 The Effect of Particle Size on Reaction Rate Lab Reaction Rate Reaction rates are therefore determined by. During this experiment we actively tried to answer the question; Did the amount of catalyst affect the reaction rate? Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. Study with quizlet and memorize. With the obtained data, it is possible to. Lab Reaction Rate.
From www.youtube.com
Calculating Rates of Reaction (2016) IB Biology YouTube Lab Reaction Rate What follows is general guidance and. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. The rate law of a chemical reaction is a mathematical. Lab Reaction Rate.
From studylib.net
Lab pg1 (Reaction Rate) Lab Reaction Rate Reaction rates are therefore determined by. The rate of reaction is the change in the amount of a reactant or product per unit time. Reaction rates are therefore determined by measuring the time. In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. Before physically performing a reaction in a. Lab Reaction Rate.
From www.slideshare.net
TOPIC 5. RATE OF REACTIONLAB Lab Reaction Rate In this lab we explored the rate of a chemical reaction at different temperatures and with different reactant particle sizes. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Study with quizlet and memorize. During this experiment we actively tried to answer the question; The reaction rate would be slower than the rate. Lab Reaction Rate.
From about.dataclassroom.com
Reaction Rate Lab — DataClassroom Lab Reaction Rate Reaction rates are therefore determined by measuring the time. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Copper(ii) sulfate increase the reaction time, the presence of cuso4 provides an alternate where the reaction can take place with lower activation. The rate of reaction is the change in the amount of a reactant. Lab Reaction Rate.
From www.studypool.com
SOLUTION Chemistry b unit 1 lab reaction rate student guide Studypool Lab Reaction Rate The reaction rate would be slower than the rate at 95°c. Study with quizlet and memorize. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. Did the amount of catalyst affect the reaction rate? Reaction rates are therefore determined by. In. Lab Reaction Rate.
From www.chegg.com
Solved LAB 3Factors Affecting Reaction Rate INTRODUCTION. Lab Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. What follows is general guidance and. The rate of reaction is the change in the amount of a reactant or product per unit time. With the obtained data, it is possible to calculate the reaction rate either algebraically or graphically. Before physically. Lab Reaction Rate.