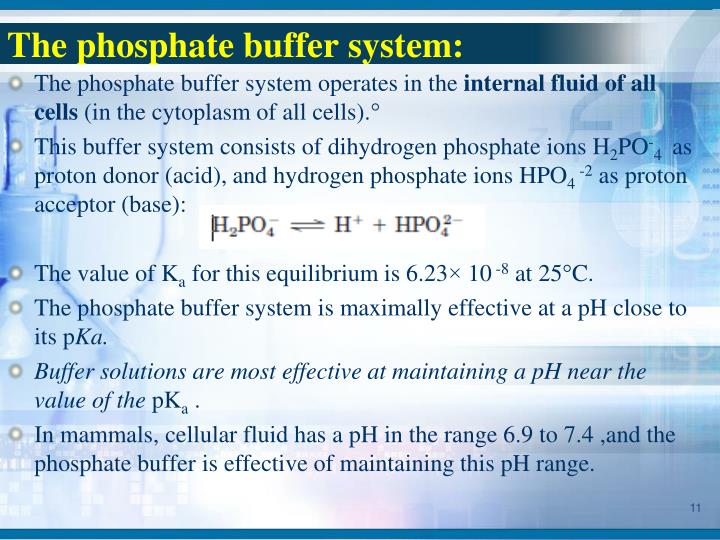
What Is Buffer In Human Body . Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffer systems work by neutralising added acid or base to resist changes to ph. It takes only seconds for the chemical buffers in the blood to make. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. Buffers are substances that help maintain the ph of a solution within a specific range. Other buffer systems in the human body include the phosphate buffer. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph.
from www.slideserve.com
The buffer systems in the human body are extremely efficient, and different systems work at different rates. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are solutions that contain a weak acid and its a conjugate base; When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Other buffer systems in the human body include the phosphate buffer. Buffer systems work by neutralising added acid or base to resist changes to ph. It takes only seconds for the chemical buffers in the blood to make. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. Buffers are substances that help maintain the ph of a solution within a specific range. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph.
PPT Buffers of Biological & Clinical Significance PowerPoint
What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. Buffer systems work by neutralising added acid or base to resist changes to ph. It takes only seconds for the chemical buffers in the blood to make. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffers are substances that help maintain the ph of a solution within a specific range. Buffers are solutions that contain a weak acid and its a conjugate base; Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Other buffer systems in the human body include the phosphate buffer.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog What Is Buffer In Human Body Buffers are solutions that contain a weak acid and its a conjugate base; Buffer systems work by neutralising added acid or base to resist changes to ph. It takes only seconds for the chemical buffers in the blood to make. Other buffer systems in the human body include the phosphate buffer. When h + is low, or excess base is. What Is Buffer In Human Body.
From www.slideshare.net
Buffer system What Is Buffer In Human Body Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to make. Buffers are substances that help maintain the ph of a solution within a specific range. Other buffer. What Is Buffer In Human Body.
From slideplayer.com
Biology I What is pH?. ppt download What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are substances that help maintain the ph of a solution within a specific range. The buffer systems in the human body are extremely efficient, and different systems work at. What Is Buffer In Human Body.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free What Is Buffer In Human Body Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. For example, when h + is added, the buffer system acts to ‘mop up’ excess h. What Is Buffer In Human Body.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. Other buffer systems in the human body include the phosphate buffer. It. What Is Buffer In Human Body.
From www.youtube.com
Acid Base Physiology Part One Basics Buffers Renal Physiology What Is Buffer In Human Body Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations. What Is Buffer In Human Body.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body What Is Buffer In Human Body For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are substances that help maintain the ph of a solution within a specific range. Buffer systems work by neutralising added acid or base to resist changes to ph. Buffers are solutions that contain a weak acid and its a conjugate base; They. What Is Buffer In Human Body.
From www.youtube.com
Buffer action in the blood YouTube What Is Buffer In Human Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. It takes only seconds for the chemical buffers in. What Is Buffer In Human Body.
From www.pinterest.com
chemical and physiological pH buffers Biochemistry notes, Nursing What Is Buffer In Human Body Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. The buffer systems in the human body are extremely efficient, and different systems work at different rates. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are substances that help maintain. What Is Buffer In Human Body.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is Buffer In Human Body For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are substances that help maintain the ph of a solution within a specific range. Buffers are solutions that contain a weak acid and its a conjugate base; They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The. What Is Buffer In Human Body.
From www.slideshare.net
Buffer system What Is Buffer In Human Body Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. It takes only seconds for the chemical buffers in the blood to make. Other buffer systems in the. What Is Buffer In Human Body.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffers are solutions that contain a weak acid and its a conjugate base; For example, when h +. What Is Buffer In Human Body.
From www.slideserve.com
PPT 1. Body Fluid Compartments PowerPoint Presentation, free download What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Other buffer systems in the human body include the phosphate buffer. Buffers are substances that. What Is Buffer In Human Body.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. Other buffer systems in the human body include the phosphate buffer. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. For example, when h + is added, the. What Is Buffer In Human Body.
From beautymedicaldevices.com
What is Miracle Body Buffer and the Benefits of this Ultimate Body Tool? What Is Buffer In Human Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to make. Buffer systems work by neutralising added acid or base to resist changes to ph. Other buffer systems in the human body include the phosphate buffer. Buffers are solutions that contain. What Is Buffer In Human Body.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The buffer systems in the human body are extremely efficient, and different systems work at different rates. For example, when h + is added, the buffer system acts to ‘mop up’ excess. What Is Buffer In Human Body.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching What Is Buffer In Human Body For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are solutions that contain a weak acid and its a conjugate base; Buffers are substances that help maintain the ph of a solution within a specific range. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Describe. What Is Buffer In Human Body.
From www.slideshare.net
Buffers in the body What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. Buffer systems work by neutralising added acid or base to resist changes to ph. Buffers are substances that help maintain the ph of a solution within a specific range. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations. What Is Buffer In Human Body.
From joirryihd.blob.core.windows.net
What Are The Buffers In The Body at Frank Stookey blog What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. Buffers are substances that help maintain the ph of a solution within a specific range. Buffer systems work by neutralising added acid or base to resist changes to ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h +. What Is Buffer In Human Body.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important To Homeostasis What Is Buffer In Human Body Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffers are substances that help maintain the ph of a solution within a specific range. Other. What Is Buffer In Human Body.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is Buffer In Human Body They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Buffers are substances that help maintain the ph of a solution within a specific range. It takes only. What Is Buffer In Human Body.
From dxorrxiwe.blob.core.windows.net
Buffer In Body Fluids at Judith Grunwald blog What Is Buffer In Human Body Buffer systems work by neutralising added acid or base to resist changes to ph. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. The. What Is Buffer In Human Body.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Is Buffer In Human Body For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are substances that help maintain the ph of a solution within a specific range. Buffers are solutions that contain a weak acid and its a conjugate base; It takes only seconds for the chemical buffers in the blood to make. The buffer. What Is Buffer In Human Body.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. It takes only seconds for the chemical buffers in the blood to make. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. For example, when h +. What Is Buffer In Human Body.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is Buffer In Human Body They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The buffer systems in the human body are extremely efficient, and different systems work at different rates. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. When h + is low, or excess base is added, the buffer. What Is Buffer In Human Body.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. For example, when h + is added, the buffer system acts to. What Is Buffer In Human Body.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts What Is Buffer In Human Body Other buffer systems in the human body include the phosphate buffer. Buffer systems work by neutralising added acid or base to resist changes to ph. The buffer systems in the human body are extremely efficient, and different systems work at different rates. When h + is low, or excess base is added, the buffer can ‘donate’ its own h +. What Is Buffer In Human Body.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is Buffer In Human Body Other buffer systems in the human body include the phosphate buffer. When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Buffer systems work by neutralising added acid or base to resist changes to ph. The buffer systems in the human body. What Is Buffer In Human Body.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Is Buffer In Human Body Buffers are solutions that contain a weak acid and its a conjugate base; They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffer systems work by neutralising added acid or base to resist changes to ph. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Other buffer. What Is Buffer In Human Body.
From www.pinterest.ca
(MCAT favorite) VHY Bicarbonate Buffer System Medical student What Is Buffer In Human Body Other buffer systems in the human body include the phosphate buffer. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffer systems work by neutralising added acid or base to resist changes to ph. Buffers are solutions that contain a weak acid and its a conjugate base; It takes only seconds for the chemical buffers. What Is Buffer In Human Body.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is Buffer In Human Body Buffer systems work by neutralising added acid or base to resist changes to ph. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. Buffers are solutions that contain a weak acid and. What Is Buffer In Human Body.
From www.slideshare.net
02 hydrolysis. buffers__colloids What Is Buffer In Human Body When h + is low, or excess base is added, the buffer can ‘donate’ its own h + to the solution to try and minimise the ph change. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. Buffer systems work by neutralising added acid or base to resist changes. What Is Buffer In Human Body.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. Describe the major buffer systems of the body (e.g., bicarbonate buffer system, protein buffer system) and their locations (e.g., extracellular. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. When h + is low, or excess base is added,. What Is Buffer In Human Body.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Is Buffer In Human Body It takes only seconds for the chemical buffers in the blood to make. For example, when h + is added, the buffer system acts to ‘mop up’ excess h +. Buffers are solutions that contain a weak acid and its a conjugate base; They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The buffer systems. What Is Buffer In Human Body.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is Buffer In Human Body Buffers are substances that help maintain the ph of a solution within a specific range. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to make. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. When. What Is Buffer In Human Body.