Threshold Energy Is Equal To . We can use the law of conservation of energy and momentum to. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. what exactly is the definition of threshold energy for a reaction ? X + x → y + y. Is it the energy that a should have so that so that the heavier of. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the.
from www.doubtnut.com
Is it the energy that a should have so that so that the heavier of. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. We can use the law of conservation of energy and momentum to. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. X + x → y + y. what exactly is the definition of threshold energy for a reaction ?
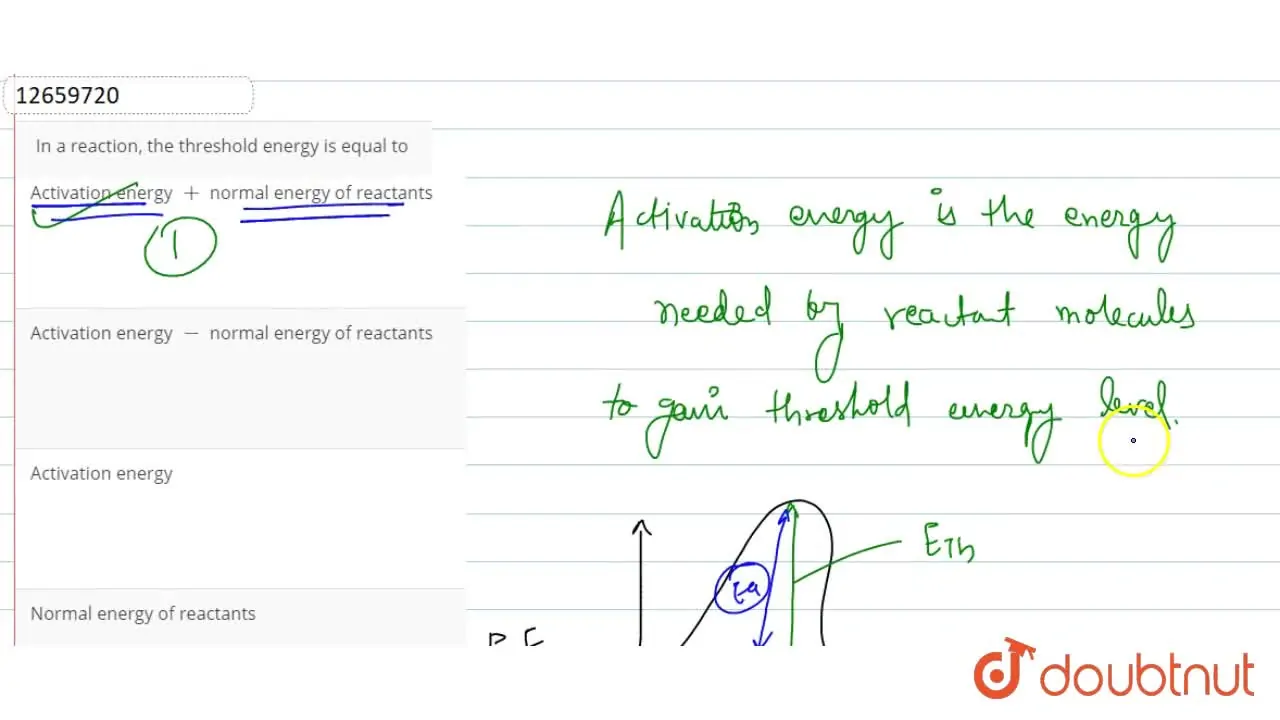
In a reaction, the threshold energy is equal to
Threshold Energy Is Equal To X + x → y + y. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. Is it the energy that a should have so that so that the heavier of. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. We can use the law of conservation of energy and momentum to. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. what exactly is the definition of threshold energy for a reaction ?
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy Is Equal To The minimum energy that all colliding molecules must possess in order to make the collisions effective and. We can use the law of conservation of energy and momentum to. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. Is it the energy that a should have so that so that the. Threshold Energy Is Equal To.
From www.slideserve.com
PPT 1.2 Radiation and Quantum Phenomena Quantum Threshold Energy Is Equal To The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. Is it the energy that a should have so that so that the heavier of. We can use the law of conservation of energy and momentum to. the minimum energy that molecules need to have in order for a reaction to. Threshold Energy Is Equal To.
From www.sarthaks.com
The energy profile diagram for the reaction `CO(g)+NO_(2)(g) hArr CO Threshold Energy Is Equal To the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy. Threshold Energy Is Equal To.
From www.youtube.com
Calculate threshold frequency Dual nature of light Physics Khan Threshold Energy Is Equal To The minimum energy that all colliding molecules must possess in order to make the collisions effective and. X + x → y + y. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. Is it the energy that a should have so that so that the heavier of. activation energy. Threshold Energy Is Equal To.
From www.youtube.com
Explain the terms Threshold energy 12 CHEMICAL Threshold Energy Is Equal To X + x → y + y. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. We can use the law of conservation of energy and momentum. Threshold Energy Is Equal To.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy Is Equal To We can use the law of conservation of energy and momentum to. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. Is it the. Threshold Energy Is Equal To.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Is Equal To the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. X + x → y + y. what exactly is the definition of threshold energy for a reaction ? activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers. Threshold Energy Is Equal To.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Threshold Energy Is Equal To the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. We can use the law of conservation of energy and momentum to. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to. Threshold Energy Is Equal To.
From www.numerade.com
SOLVEDDetermine the Q value and the threshold energy for 8^16 O+ 0^1 Threshold Energy Is Equal To Is it the energy that a should have so that so that the heavier of. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. We can use the law of. Threshold Energy Is Equal To.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energy Is Equal To The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The minimum energy that all colliding molecules must possess in order to make the collisions. Threshold Energy Is Equal To.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy Threshold Energy Is Equal To the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. We can use the law of conservation of energy and momentum to. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. what exactly is the definition of threshold. Threshold Energy Is Equal To.
From www.doubtnut.com
Threshold energy is equal to Threshold Energy Is Equal To what exactly is the definition of threshold energy for a reaction ? activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. X + x → y + y. We can use. Threshold Energy Is Equal To.
From exogobhlj.blob.core.windows.net
Threshold Energy For Proton at Irene Browning blog Threshold Energy Is Equal To We can use the law of conservation of energy and momentum to. what exactly is the definition of threshold energy for a reaction ? the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. X + x → y + y. The threshold frequency in a chemical. Threshold Energy Is Equal To.
From www.researchgate.net
Variations of remaining energy and threshold energy against prediction Threshold Energy Is Equal To Is it the energy that a should have so that so that the heavier of. X + x → y + y. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. the minimum energy that molecules need to have in order for a reaction to take place. Threshold Energy Is Equal To.
From www.numerade.com
SOLVEDFor producing the effective collisions, the colliding molecules Threshold Energy Is Equal To The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. what exactly is the definition of threshold energy for a reaction ? the minimum energy that molecules need to have. Threshold Energy Is Equal To.
From www.doubtnut.com
The Threshold energy is given as E(0) and radiation of energy E Threshold Energy Is Equal To activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the.. Threshold Energy Is Equal To.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Energy Is Equal To Is it the energy that a should have so that so that the heavier of. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. We can use the law of conservation of energy and momentum to. threshold energy= average of the initial kinetic energy possessed by. Threshold Energy Is Equal To.
From www.youtube.com
Nuclear Physics (Qvalue, Threshold Energy) YouTube Threshold Energy Is Equal To The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Is. Threshold Energy Is Equal To.
From www.researchgate.net
Same as Fig. 12 but using a detector with threshold energy equal to 20 Threshold Energy Is Equal To The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. The. Threshold Energy Is Equal To.
From www.researchgate.net
Same as Fig. 10 but using a detector with threshold energy equal to 20 Threshold Energy Is Equal To We can use the law of conservation of energy and momentum to. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The threshold frequency in. Threshold Energy Is Equal To.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Is Equal To The minimum energy that all colliding molecules must possess in order to make the collisions effective and. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while. Threshold Energy Is Equal To.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry Threshold Energy Is Equal To activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. We can use the law of conservation of energy and momentum to. Is it the energy that a. Threshold Energy Is Equal To.
From www.researchgate.net
An example of the threshold energy determination for aC sample Threshold Energy Is Equal To threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Is it the energy that a should have so that so that the heavier of. The threshold frequency in. Threshold Energy Is Equal To.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Is Equal To We can use the law of conservation of energy and momentum to. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. X + x → y + y.. Threshold Energy Is Equal To.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Is Equal To the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. We can use the law of conservation of energy and momentum to. Is it the energy that a should have so that so that the heavier of. threshold energy= average of the initial kinetic energy possessed by. Threshold Energy Is Equal To.
From www.doubtnut.com
In a reaction, the threshold energy is equal to Threshold Energy Is Equal To Is it the energy that a should have so that so that the heavier of. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. X + x → y. Threshold Energy Is Equal To.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Is Equal To threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. Is it the energy that a should have so that so that the heavier of. The threshold. Threshold Energy Is Equal To.
From www.researchgate.net
The threshold energy, √ s th , for K + Λ and K + Σ Download Threshold Energy Is Equal To threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. Is it. Threshold Energy Is Equal To.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy Is Equal To activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy.. Threshold Energy Is Equal To.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy Is Equal To what exactly is the definition of threshold energy for a reaction ? activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. We can use the law of conservation of energy. Threshold Energy Is Equal To.
From www.researchgate.net
(a) Energy (Er in a.u.) and the threshold energy (E2s in a.u.) vs. 1 Z Threshold Energy Is Equal To X + x → y + y. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. the minimum energy that molecules need to have in order for a reaction to take place is. Threshold Energy Is Equal To.
From www.youtube.com
Explain Q Value and, Threshold energy Nuclear Chemistry Physical Threshold Energy Is Equal To activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. Is it the energy that a should have so that so that the heavier of. The minimum energy that all colliding molecules must possess in order to make the collisions effective and. the minimum energy that molecules need to. Threshold Energy Is Equal To.
From www.researchgate.net
(PDF) Threshold Energy Threshold Energy Is Equal To the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. X + x → y + y. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The threshold frequency in a chemical reaction is the minimum energy. Threshold Energy Is Equal To.
From www.researchgate.net
The threshold energy E 0 is determined by the equation (3.1). The red Threshold Energy Is Equal To Is it the energy that a should have so that so that the heavier of. The threshold frequency in a chemical reaction is the minimum energy that molecules need to have for the. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. We can use the law of conservation. Threshold Energy Is Equal To.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy Is Equal To Is it the energy that a should have so that so that the heavier of. threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at. activation energy represents the minimum energy required for a chemical reaction to occur, while threshold energy refers to the. The minimum energy that. Threshold Energy Is Equal To.