Vibrational Frequency In Chemistry . Reduced mass of the diatomic molecule μ. calculation of vibrational frequencies. For a simple harmonic oscillator the period r is given by: a wavenumber is often used due to its direct relationship with both frequency and energy. Note that v and ν look. Where k is the force constant. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are.
from openmopac.net
a wavenumber is often used due to its direct relationship with both frequency and energy. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. Where k is the force constant. Note that v and ν look. calculation of vibrational frequencies. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. For a simple harmonic oscillator the period r is given by: Reduced mass of the diatomic molecule μ.
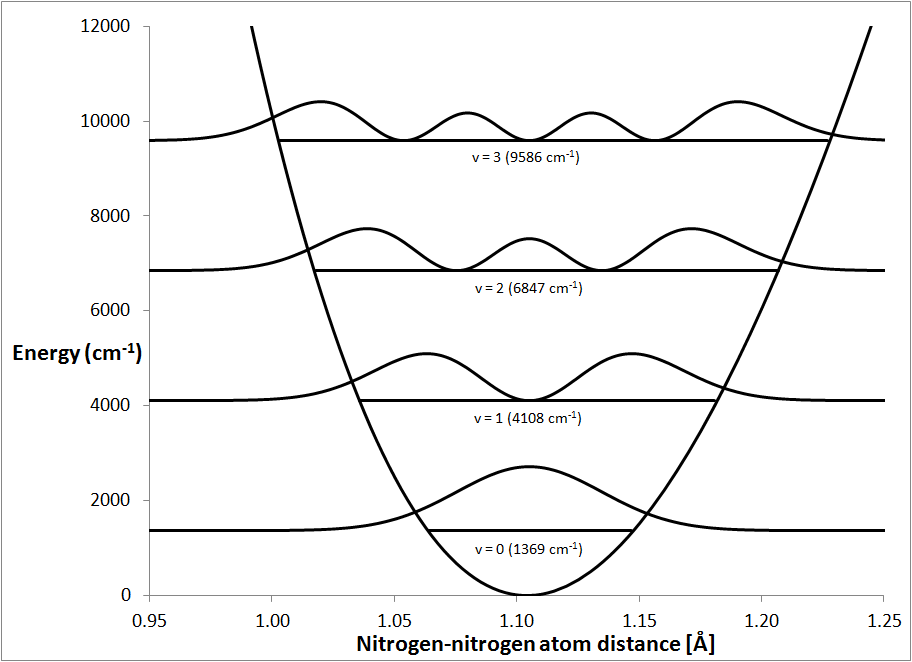
Vibrational quantities Quantum versus Classical description
Vibrational Frequency In Chemistry Reduced mass of the diatomic molecule μ. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. For a simple harmonic oscillator the period r is given by: Where k is the force constant. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. calculation of vibrational frequencies. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. Reduced mass of the diatomic molecule μ. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. a wavenumber is often used due to its direct relationship with both frequency and energy. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. Note that v and ν look.
From www.youtube.com
Calculation of Vibrational Frequency Hooke's Law (1) YouTube Vibrational Frequency In Chemistry vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. Where k is the force constant. Note that v and ν look. For a simple harmonic oscillator the period r is given. Vibrational Frequency In Chemistry.
From www.masterorganicchemistry.com
Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model Vibrational Frequency In Chemistry the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. Where k is the force constant. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. a wavenumber is often used due to its direct relationship with both. Vibrational Frequency In Chemistry.
From chem.libretexts.org
13.5 Vibrational Overtones Chemistry LibreTexts Vibrational Frequency In Chemistry these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e,. Vibrational Frequency In Chemistry.
From brokeasshome.com
tables of molecular vibrational frequencies Vibrational Frequency In Chemistry Where k is the force constant. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. Reduced mass of the diatomic molecule μ. calculation of vibrational frequencies. For a simple harmonic oscillator the period r is given by: vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such. Vibrational Frequency In Chemistry.
From hydrogenatomgirikosa.blogspot.com
Hydrogen Atom Hydrogen Atom Vibration Frequency Vibrational Frequency In Chemistry Note that v and ν look. For a simple harmonic oscillator the period r is given by: vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. these complex vibrations can be broken down. Vibrational Frequency In Chemistry.
From www.researchgate.net
(a) Vibrational spectra of different modes of Cu and graphene atoms at Vibrational Frequency In Chemistry vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. calculation of vibrational frequencies. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and. Vibrational Frequency In Chemistry.
From www.researchgate.net
Illustration of vibrational transitions of various molecules with Vibrational Frequency In Chemistry a wavenumber is often used due to its direct relationship with both frequency and energy. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. Where k is the force constant. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such. Vibrational Frequency In Chemistry.
From dxobtwzsk.blob.core.windows.net
Vibrational Frequency Chemistry at Anna Sheppard blog Vibrational Frequency In Chemistry these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. Reduced mass of the diatomic molecule μ. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. Note that v and ν look. a wavenumber is often used due to its direct relationship. Vibrational Frequency In Chemistry.
From pubs.acs.org
Vibrational SumFrequency Generation Spectroscopy in the Energy Vibrational Frequency In Chemistry calculation of vibrational frequencies. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. a wavenumber is often used due to its direct relationship with both frequency and energy. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. Where k is the. Vibrational Frequency In Chemistry.
From studylib.net
Vibrational frequencies Vibrational Frequency In Chemistry vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. calculation of vibrational frequencies. For a simple harmonic oscillator the period r is given by: A normal mode is a molecular vibration where some or all atoms vibrate together with the same. the vibrational frequency expressed in wavenumber units c. Vibrational Frequency In Chemistry.
From www.researchgate.net
Vibrational frequencies obtained from the FTIR results at different Vibrational Frequency In Chemistry the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e,. Vibrational Frequency In Chemistry.
From www.researchgate.net
The three vibrational modes of the water molecule and their fundamental Vibrational Frequency In Chemistry Where k is the force constant. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. calculation of vibrational frequencies. Reduced mass of the diatomic molecule μ. . Vibrational Frequency In Chemistry.
From openmopac.net
Vibrational quantities Quantum versus Classical description Vibrational Frequency In Chemistry vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. Where k is the force constant. calculation of vibrational frequencies. these complex vibrations. Vibrational Frequency In Chemistry.
From www.researchgate.net
(a) Effective rotational constant and (b) vibrational frequency of the Vibrational Frequency In Chemistry For a simple harmonic oscillator the period r is given by: calculation of vibrational frequencies. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. a wavenumber is often used due to its direct relationship with both frequency and energy. Note that v and ν look. Where k is the force. Vibrational Frequency In Chemistry.
From www.researchgate.net
Schematic representation of vibrational modes of the H 2 O 2 isolated Vibrational Frequency In Chemistry Where k is the force constant. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. For a simple harmonic oscillator the period r is given by: calculation of vibrational frequencies. Reduced mass of. Vibrational Frequency In Chemistry.
From www.researchgate.net
(PDF) Cavity frequencydependent theory for vibrational polariton chemistry Vibrational Frequency In Chemistry Reduced mass of the diatomic molecule μ. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. For a simple harmonic oscillator the period r is given by:. Vibrational Frequency In Chemistry.
From pubs.acs.org
VIBFREQ1295 A New Database for Vibrational Frequency Calculations Vibrational Frequency In Chemistry Reduced mass of the diatomic molecule μ. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. For a simple harmonic oscillator the period r is given by: the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c. Vibrational Frequency In Chemistry.
From www.researchgate.net
Vibration frequencies of the main functions encountered in organic Vibrational Frequency In Chemistry For a simple harmonic oscillator the period r is given by: a wavenumber is often used due to its direct relationship with both frequency and energy. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. A normal mode is a molecular vibration where some or all atoms vibrate together with. Vibrational Frequency In Chemistry.
From dxooaxggc.blob.core.windows.net
Vibration And Frequency Relation at Arline Shirley blog Vibrational Frequency In Chemistry the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. calculation of vibrational frequencies. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. these complex vibrations can be broken down mathematically into individual vibrational modes, a. Vibrational Frequency In Chemistry.
From www.researchgate.net
Calculated IR vibrational frequencies of the complexes and their Vibrational Frequency In Chemistry A normal mode is a molecular vibration where some or all atoms vibrate together with the same. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. a wavenumber is often used due to its direct relationship with both frequency and energy. Where k is. Vibrational Frequency In Chemistry.
From www.researchgate.net
Vibration frequencies (cm 1 ) in IR spectra of CuPc* Download Table Vibrational Frequency In Chemistry Reduced mass of the diatomic molecule μ. Note that v and ν look. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. A normal mode is a. Vibrational Frequency In Chemistry.
From www.researchgate.net
Plot of calculated 1 HNMR chemical shifts of H A vs. calculated Vibrational Frequency In Chemistry vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. For a simple harmonic oscillator the period r is given by: Reduced mass of the diatomic molecule μ. Note that v and ν look. A normal mode is a molecular vibration where some or all atoms vibrate together with. Vibrational Frequency In Chemistry.
From www.researchgate.net
7 Various modes of bending vibration in methylene group (CH 2 ). The Vibrational Frequency In Chemistry vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. Where k is the. Vibrational Frequency In Chemistry.
From www.researchgate.net
Variation of the vibration frequency with respect to the material Vibrational Frequency In Chemistry Note that v and ν look. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. a wavenumber is often used due to its direct relationship with both frequency and energy. Reduced mass of the diatomic molecule μ. calculation of vibrational frequencies. A normal mode is a molecular vibration where. Vibrational Frequency In Chemistry.
From openmopac.net
Vibrational quantities Quantum versus Classical description Vibrational Frequency In Chemistry these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. Where k is the force constant. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. calculation of vibrational frequencies. a wavenumber is often used due to its direct relationship with both. Vibrational Frequency In Chemistry.
From www.researchgate.net
Calculated and experimental vibrational frequency spectra (FTIR) of Vibrational Frequency In Chemistry Where k is the force constant. Note that v and ν look. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. vibrational frequency refers to the rate at which atoms in a molecule oscillate about their equilibrium positions. a wavenumber is often used. Vibrational Frequency In Chemistry.
From pubs.acs.org
Vibrational SumFrequency Generation Spectroscopy at the Water/Lipid Vibrational Frequency In Chemistry Reduced mass of the diatomic molecule μ. Note that v and ν look. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. a wavenumber is often used due to its direct relationship with both frequency and energy. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules,. Vibrational Frequency In Chemistry.
From chem.libretexts.org
12 Vibrational Spectroscopy of Diatomic Molecules Chemistry LibreTexts Vibrational Frequency In Chemistry the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. Reduced mass of the diatomic molecule μ. Note that v and ν look. A normal mode is a. Vibrational Frequency In Chemistry.
From courses.lumenlearning.com
Infrared Spectroscopy MCC Organic Chemistry Vibrational Frequency In Chemistry the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. a wavenumber is often used due to its direct relationship with both frequency and energy. Where k is the force constant. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such. Vibrational Frequency In Chemistry.
From www.researchgate.net
The vibrational frequencies (cm 1 ) of the reactants, intermediates Vibrational Frequency In Chemistry calculation of vibrational frequencies. Note that v and ν look. the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. a wavenumber is often used due to its direct relationship with both frequency and energy. vibrational frequency refers to the characteristic modes of. Vibrational Frequency In Chemistry.
From www.researchgate.net
DFT calculations of vibrational frequencies (IR) of the pure MP (a) and Vibrational Frequency In Chemistry Note that v and ν look. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. Reduced mass of the diatomic molecule μ. calculation of vibrational frequencies. vibrational frequency refers to the rate. Vibrational Frequency In Chemistry.
From dxobtwzsk.blob.core.windows.net
Vibrational Frequency Chemistry at Anna Sheppard blog Vibrational Frequency In Chemistry a wavenumber is often used due to its direct relationship with both frequency and energy. vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. Reduced mass of the diatomic molecule μ. For a simple harmonic oscillator the period r is given by: calculation of vibrational frequencies.. Vibrational Frequency In Chemistry.
From dxobtwzsk.blob.core.windows.net
Vibrational Frequency Chemistry at Anna Sheppard blog Vibrational Frequency In Chemistry A normal mode is a molecular vibration where some or all atoms vibrate together with the same. these complex vibrations can be broken down mathematically into individual vibrational modes, a few of which are. calculation of vibrational frequencies. For a simple harmonic oscillator the period r is given by: vibrational frequency refers to the characteristic modes of. Vibrational Frequency In Chemistry.
From sites.cns.utexas.edu
Normal Modes of Vibration CH 431 Chemistry Vibrational Frequency In Chemistry Reduced mass of the diatomic molecule μ. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. For a simple harmonic oscillator the period r is given by: the vibrational frequency expressed in wavenumber units c m − 1 units is ν ~ = 1 2 π c k / μ. . Vibrational Frequency In Chemistry.
From dxobtwzsk.blob.core.windows.net
Vibrational Frequency Chemistry at Anna Sheppard blog Vibrational Frequency In Chemistry vibrational frequency refers to the characteristic modes of vibration exhibited by molecules, such as a1, a2, e, and e' in. Where k is the force constant. A normal mode is a molecular vibration where some or all atoms vibrate together with the same. For a simple harmonic oscillator the period r is given by: Reduced mass of the diatomic. Vibrational Frequency In Chemistry.