How Is Hess's Law Applied In Calculating Enthalpy . If a process can be written as the sum of several. Hess’s law is also known as hess’s. hess’s law states that the enthalpy change for a reaction is independent of the path taken. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. calculate the molar enthalpy of formation from combustion data using hess's law; Using the enthalpy of formation, calculate the unknown. this type of calculation usually involves the use of hess’s law, which states:
from www.youtube.com
the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. this type of calculation usually involves the use of hess’s law, which states: If a process can be written as the sum of several. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. hess’s law states that the enthalpy change for a reaction is independent of the path taken. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. Using the enthalpy of formation, calculate the unknown. Hess’s law is also known as hess’s. calculate the molar enthalpy of formation from combustion data using hess's law;
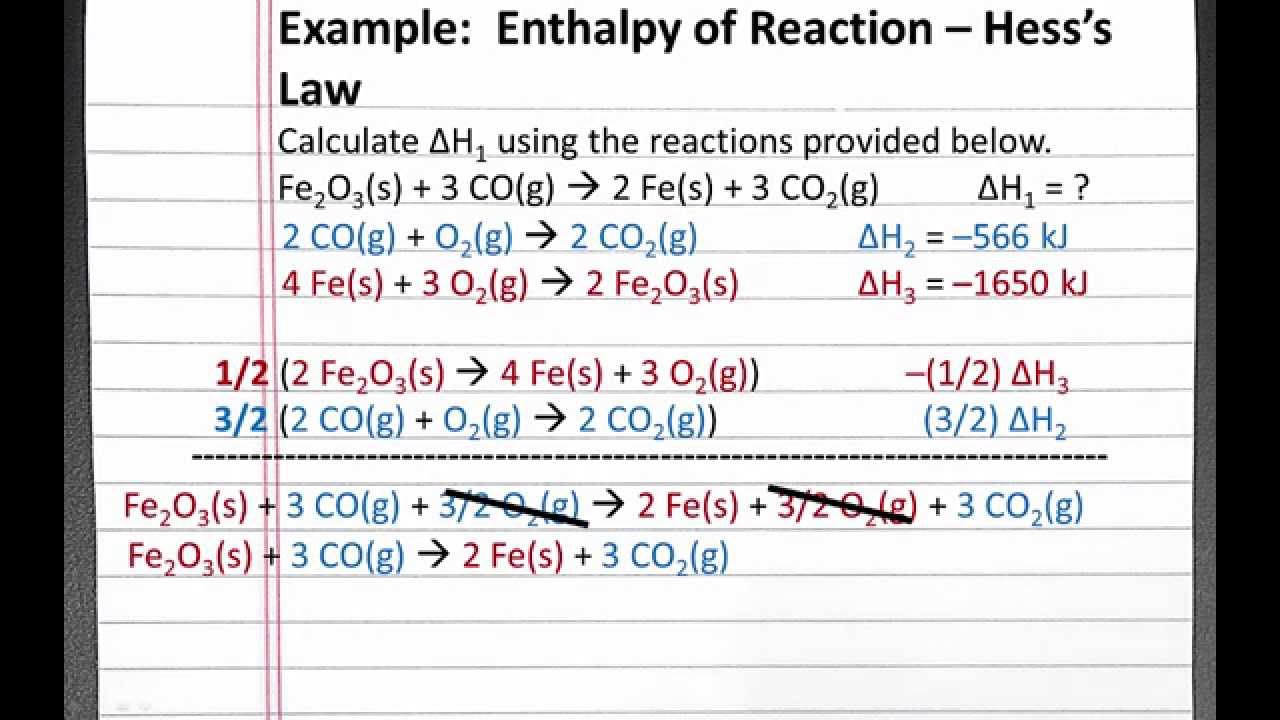
CHEMISTRY 101 Enthalpy of Reaction, Hess's Law YouTube
How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction is independent of the path taken. calculate the molar enthalpy of formation from combustion data using hess's law; hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. this type of calculation usually involves the use of hess’s law, which states: If a process can be written as the sum of several. Hess’s law is also known as hess’s. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation ID456765 How Is Hess's Law Applied In Calculating Enthalpy this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. Hess’s law is also known as hess’s. hess’s law states that. How Is Hess's Law Applied In Calculating Enthalpy.
From www.studocu.com
Hess’s law Dr. Andy Miller HEH't LAW calculating Enthalpy changes using Hess's Law Hess's How Is Hess's Law Applied In Calculating Enthalpy Hess’s law is also known as hess’s. If a process can be written as the sum of several. hess’s law states that the enthalpy change for a reaction is independent of the path taken. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction,. How Is Hess's Law Applied In Calculating Enthalpy.
From www.numerade.com
SOLVED 'experimentully determined heats (10) Apply Hess' Law t0 find the Heat of Formation for How Is Hess's Law Applied In Calculating Enthalpy Using the enthalpy of formation, calculate the unknown. calculate the molar enthalpy of formation from combustion data using hess's law; the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess's law of constant heat summation (or just. How Is Hess's Law Applied In Calculating Enthalpy.
From slidetodoc.com
Section 15 4 Calculating Enthalpy Change Apply Hesss How Is Hess's Law Applied In Calculating Enthalpy Using the enthalpy of formation, calculate the unknown. calculate the molar enthalpy of formation from combustion data using hess's law; Hess’s law is also known as hess’s. If a process can be written as the sum of several. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction,. How Is Hess's Law Applied In Calculating Enthalpy.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent How Is Hess's Law Applied In Calculating Enthalpy calculate the molar enthalpy of formation from combustion data using hess's law; this type of calculation usually involves the use of hess’s law, which states: hess’s law states that the enthalpy change for a reaction is independent of the path taken. Hess’s law is also known as hess’s. the formation of co 2 (g) from its. How Is Hess's Law Applied In Calculating Enthalpy.
From www.solutionspile.com
[Solved] (a) Use Hess's Law to calculate the enthalpy How Is Hess's Law Applied In Calculating Enthalpy hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. calculate the molar enthalpy of formation from combustion data using hess's law; this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation. How Is Hess's Law Applied In Calculating Enthalpy.
From www.slideserve.com
PPT Thermochemisty (Enthalpy) and Hess’s Law PowerPoint Presentation ID3527437 How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. hess’s law states that the enthalpy change for a reaction is independent of the path taken. Using the enthalpy of formation, calculate the unknown. this type of calculation usually involves the use of hess’s law, which states: calculate the molar enthalpy of formation from combustion data. How Is Hess's Law Applied In Calculating Enthalpy.
From www.slideserve.com
PPT Hess’ Law of Heat Summation PowerPoint Presentation, free download ID2509342 How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. Hess’s law is also known as hess’s. If a process can be written as the sum. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess's Law Problems & Enthalpy Change Chemistry YouTube How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. hess’s law states that the enthalpy change for a reaction is independent of the path taken. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. calculate the molar enthalpy of formation. How Is Hess's Law Applied In Calculating Enthalpy.
From www.chegg.com
Solved 5) Using Hess' Law, calculate the change in enthalpy How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: hess’s law states that the enthalpy change for a reaction is independent of the path taken. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. Using the enthalpy of. How Is Hess's Law Applied In Calculating Enthalpy.
From www.chegg.com
Solved Ex 7.14 Hess's Law Calculate the enthalpy change for How Is Hess's Law Applied In Calculating Enthalpy calculate the molar enthalpy of formation from combustion data using hess's law; hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. Using the enthalpy of formation, calculate the unknown. If a process can be written as the sum of several. the formation. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess's Law to calculate enthalpy change of a chemical reaction YouTube How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. calculate the molar enthalpy of formation from combustion data using hess's law; the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. this page explains hess's law,. How Is Hess's Law Applied In Calculating Enthalpy.
From www.numerade.com
SOLVED Use Hess's Law to calculate the enthalpy change for the reaction from the following data How Is Hess's Law Applied In Calculating Enthalpy this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess’s law states that the enthalpy. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess's Law Chemistry Tutorial YouTube How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: If a process can be written as the sum of several. Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction is independent of the path taken. the formation of co 2 (g) from its elements. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess's Law Calculations (Enthalpy of Combustion) YouTube How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: If a process can be written as the sum of several. calculate the molar enthalpy of formation from combustion data using hess's law; hess’s law states that the enthalpy change for a reaction is independent of the path taken. hess's law of constant. How Is Hess's Law Applied In Calculating Enthalpy.
From www.sliderbase.com
Hess's Law Presentation Chemistry How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: If a process can be written as the sum of several. calculate the molar enthalpy of formation from combustion data using hess's law; Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction is independent of. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess Law Chemistry Problems Enthalpy Change Constant Heat of Summation Example 2 YouTube How Is Hess's Law Applied In Calculating Enthalpy calculate the molar enthalpy of formation from combustion data using hess's law; Using the enthalpy of formation, calculate the unknown. Hess’s law is also known as hess’s. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. the formation of co 2 (g) from its. How Is Hess's Law Applied In Calculating Enthalpy.
From solvedlib.com
Use Hess s Law to calculate the standard enthalpy cha… SolvedLib How Is Hess's Law Applied In Calculating Enthalpy Hess’s law is also known as hess’s. Using the enthalpy of formation, calculate the unknown. this type of calculation usually involves the use of hess’s law, which states: hess’s law states that the enthalpy change for a reaction is independent of the path taken. calculate the molar enthalpy of formation from combustion data using hess's law; . How Is Hess's Law Applied In Calculating Enthalpy.
From www.sliderbase.com
Hess's Law Presentation Chemistry How Is Hess's Law Applied In Calculating Enthalpy Hess’s law is also known as hess’s. Using the enthalpy of formation, calculate the unknown. If a process can be written as the sum of several. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. hess’s law states that the enthalpy change for. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess's Law (Determining Enthalpy of Reaction) YouTube How Is Hess's Law Applied In Calculating Enthalpy this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction is independent of the path taken. hess's law of constant heat summation (or just hess's law) states. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Calculating Enthalpy and Hess's Law YouTube How Is Hess's Law Applied In Calculating Enthalpy calculate the molar enthalpy of formation from combustion data using hess's law; this type of calculation usually involves the use of hess’s law, which states: this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. hess's law of constant heat summation (or just hess's. How Is Hess's Law Applied In Calculating Enthalpy.
From www.chegg.com
Solved Experiment 3 Data Enthalpy change Hess's law Table How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction is independent. How Is Hess's Law Applied In Calculating Enthalpy.
From study.com
Using Hess's Law to Calculate the Enthalpy of a Reaction Chemistry How Is Hess's Law Applied In Calculating Enthalpy hess’s law states that the enthalpy change for a reaction is independent of the path taken. this type of calculation usually involves the use of hess’s law, which states: calculate the molar enthalpy of formation from combustion data using hess's law; Hess’s law is also known as hess’s. this page explains hess's law, and uses it. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
How to Calculate Lattice Enthalpy Using Hess's Law (Sum of Reactions) YouTube How Is Hess's Law Applied In Calculating Enthalpy the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess’s law states that the enthalpy change for a reaction is independent of the path taken. Hess’s law is also known as hess’s. this page explains hess's law,. How Is Hess's Law Applied In Calculating Enthalpy.
From www.bartleby.com
Answered 3. Use Hess' law to calculate AH° for… bartleby How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. hess’s law states that the enthalpy change for a reaction is independent of the path taken. Using the enthalpy of formation, calculate the unknown. . How Is Hess's Law Applied In Calculating Enthalpy.
From www.numerade.com
SOLVED Experiment Enthalpy of Neutralization Use Hess' Law to Calculate the enthalpy of How Is Hess's Law Applied In Calculating Enthalpy Using the enthalpy of formation, calculate the unknown. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. this type of calculation usually involves the use of hess’s law, which states: this page explains hess's law, and uses. How Is Hess's Law Applied In Calculating Enthalpy.
From brainly.com
How is hess's law applied in calculating enthalpy How Is Hess's Law Applied In Calculating Enthalpy this type of calculation usually involves the use of hess’s law, which states: hess’s law states that the enthalpy change for a reaction is independent of the path taken. calculate the molar enthalpy of formation from combustion data using hess's law; Hess’s law is also known as hess’s. If a process can be written as the sum. How Is Hess's Law Applied In Calculating Enthalpy.
From www.science-revision.co.uk
Hess's Law How Is Hess's Law Applied In Calculating Enthalpy hess’s law states that the enthalpy change for a reaction is independent of the path taken. calculate the molar enthalpy of formation from combustion data using hess's law; hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. this page explains hess's. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
Hess' Law (Enthalpy Changes) IB Chemistry Revision Course YouTube How Is Hess's Law Applied In Calculating Enthalpy Hess’s law is also known as hess’s. hess’s law states that the enthalpy change for a reaction is independent of the path taken. If a process can be written as the sum of several. this type of calculation usually involves the use of hess’s law, which states: hess's law of constant heat summation (or just hess's law). How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
killer stratergies Hess's law for calculating enthalpy of combustion from formation for AS How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. this type of calculation usually involves the use of hess’s law, which states: Using the enthalpy of formation, calculate the unknown. hess’s law states. How Is Hess's Law Applied In Calculating Enthalpy.
From www.youtube.com
CHEMISTRY 101 Enthalpy of Reaction, Hess's Law YouTube How Is Hess's Law Applied In Calculating Enthalpy hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. Hess’s law is also known as hess’s. hess’s law states that. How Is Hess's Law Applied In Calculating Enthalpy.
From study.com
Hess's Law Definition, Diagram & Equation Lesson How Is Hess's Law Applied In Calculating Enthalpy the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. If a process can be written as the sum of several. Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction. How Is Hess's Law Applied In Calculating Enthalpy.
From www.coursehero.com
[Solved] Calculate standard enthalpy change of reaction via Hess's Law. Use... Course Hero How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or. How Is Hess's Law Applied In Calculating Enthalpy.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent How Is Hess's Law Applied In Calculating Enthalpy the formation of co 2 (g) from its elements can be thought of as occurring in two steps, which sum to the overall reaction, as described by hess’s law. hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total. calculate the molar enthalpy. How Is Hess's Law Applied In Calculating Enthalpy.
From studylib.net
HESS`S LAW and CALCULATING THE ENTHALPY OF How Is Hess's Law Applied In Calculating Enthalpy If a process can be written as the sum of several. calculate the molar enthalpy of formation from combustion data using hess's law; Using the enthalpy of formation, calculate the unknown. hess’s law states that the enthalpy change for a reaction is independent of the path taken. this type of calculation usually involves the use of hess’s. How Is Hess's Law Applied In Calculating Enthalpy.