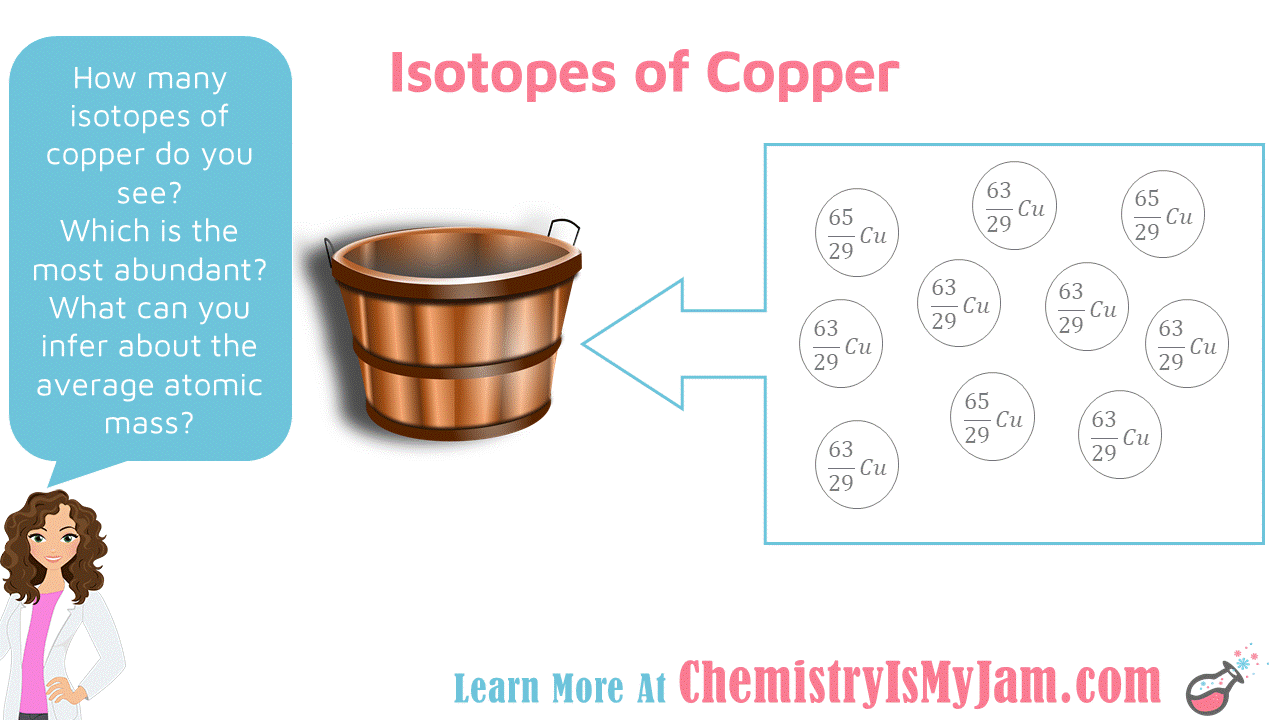
Copper Has An Average Atomic Mass Of 63.55 U . Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The relat abundance and atomic masses are 69.2% for a. You can approach this problem by using a single. As you know, the average atomic mass of an element is determined. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. A naturally occuring sample of copper consists of. ∴ the percentage of.65 29 c u isotope = 100 − x. Let the percentage of.63 29 c u isotope = x. If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: From the above data, the.
from chemistryismyjam.com
As you know, the average atomic mass of an element is determined. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. Let the percentage of.63 29 c u isotope = x. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. You can approach this problem by using a single. A naturally occuring sample of copper consists of. ∴ the percentage of.65 29 c u isotope = 100 − x. The relat abundance and atomic masses are 69.2% for a. From the above data, the. The element copper has naturally occurring isotopes with mass numbers of 63 and 65.
The Atom Chemistry Is My Jam!
Copper Has An Average Atomic Mass Of 63.55 U ∴ the percentage of.65 29 c u isotope = 100 − x. You can approach this problem by using a single. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. ∴ the percentage of.65 29 c u isotope = 100 − x. As you know, the average atomic mass of an element is determined. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. Let the percentage of.63 29 c u isotope = x. If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: The relat abundance and atomic masses are 69.2% for a. A naturally occuring sample of copper consists of. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. From the above data, the.
From ecurrencythailand.com
What Is The Atomic Mass Of Copper 63 And Copper 65? All Answers Copper Has An Average Atomic Mass Of 63.55 U If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the. Copper Has An Average Atomic Mass Of 63.55 U.
From www.youtube.com
Calculating average atomic mass of elements Tutorial YouTube Copper Has An Average Atomic Mass Of 63.55 U The element copper has naturally occurring isotopes with mass numbers of 63 and 65. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. A naturally occuring sample of copper consists of. ∴ the percentage of.65 29 c u isotope = 100 − x. You can approach this. Copper Has An Average Atomic Mass Of 63.55 U.
From www.coursehero.com
[Solved] What is the atomic mass of copper with isotopes copper63 with Copper Has An Average Atomic Mass Of 63.55 U 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. A naturally occuring sample of copper consists of. ∴ the percentage of.65 29 c u isotope = 100 − x. From. Copper Has An Average Atomic Mass Of 63.55 U.
From lessonmagiclandrace.z21.web.core.windows.net
Calculating Average Atomic Mass Worksheet Key Copper Has An Average Atomic Mass Of 63.55 U If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: As you know, the average atomic mass of an element is determined. Let the percentage of.63 29 c u isotope = x. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. From the above. Copper Has An Average Atomic Mass Of 63.55 U.
From www.bartleby.com
Answered Copper has two naturally occurring… bartleby Copper Has An Average Atomic Mass Of 63.55 U The relat abundance and atomic masses are 69.2% for a. Let the percentage of.63 29 c u isotope = x. A naturally occuring sample of copper consists of. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and. Copper Has An Average Atomic Mass Of 63.55 U.
From askfilo.com
Copper has two naturally occurring isotopes 63Cu and 65Cu. If the average.. Copper Has An Average Atomic Mass Of 63.55 U You can approach this problem by using a single. As you know, the average atomic mass of an element is determined. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relat abundance and atomic masses are 69.2% for a. From the above data, the. ∴ the percentage of.65 29 c u isotope = 100. Copper Has An Average Atomic Mass Of 63.55 U.
From cevsevml.blob.core.windows.net
Atomic Mass Number For Tin at Amy Longoria blog Copper Has An Average Atomic Mass Of 63.55 U You can approach this problem by using a single. As you know, the average atomic mass of an element is determined. If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective. Copper Has An Average Atomic Mass Of 63.55 U.
From pubchem.ncbi.nlm.nih.gov
Atomic Mass Periodic Table of Elements PubChem Copper Has An Average Atomic Mass Of 63.55 U From the above data, the. A naturally occuring sample of copper consists of. ∴ the percentage of.65 29 c u isotope = 100 − x. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. 69% for the isotope that weighs 62.93 u and 31% for the isotope. Copper Has An Average Atomic Mass Of 63.55 U.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Has An Average Atomic Mass Of 63.55 U ∴ the percentage of.65 29 c u isotope = 100 − x. The relat abundance and atomic masses are 69.2% for a. Let the percentage of.63 29 c u isotope = x. As you know, the average atomic mass of an element is determined. You can approach this problem by using a single. If we look at the periodic table,. Copper Has An Average Atomic Mass Of 63.55 U.
From hunterturninaing.blogspot.com
how to find the average atomic mass Hunter Turninaing Copper Has An Average Atomic Mass Of 63.55 U 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. A naturally occuring sample of copper consists of. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. From the above. Copper Has An Average Atomic Mass Of 63.55 U.
From askfilo.com
What mass of copper will contain 3.22×1024 atoms of copper? (Atomic mass Copper Has An Average Atomic Mass Of 63.55 U If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: From the above data, the. Let the percentage of.63 29 c u isotope = x. A naturally occuring sample of copper consists of. You can approach this problem by using a single. As you know, the average atomic mass of an element. Copper Has An Average Atomic Mass Of 63.55 U.
From www.numerade.com
SOLVED Calculate Copper has two isotopes C u63 (abundance =69.2 Copper Has An Average Atomic Mass Of 63.55 U Let the percentage of.63 29 c u isotope = x. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate). Copper Has An Average Atomic Mass Of 63.55 U.
From classfullprolonging.z14.web.core.windows.net
How To Find The Average Atomic Mass Copper Has An Average Atomic Mass Of 63.55 U As you know, the average atomic mass of an element is determined. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. The relat abundance and atomic masses are 69.2% for a. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928. Copper Has An Average Atomic Mass Of 63.55 U.
From chemistryismyjam.com
The Atom Chemistry Is My Jam! Copper Has An Average Atomic Mass Of 63.55 U Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. If we look at the periodic table, copper metal (a mixture. Copper Has An Average Atomic Mass Of 63.55 U.
From slideplayer.com
Quietly study for your quiz spelling counts! ppt download Copper Has An Average Atomic Mass Of 63.55 U A naturally occuring sample of copper consists of. As you know, the average atomic mass of an element is determined. You can approach this problem by using a single. If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%,. Copper Has An Average Atomic Mass Of 63.55 U.
From www.numerade.com
Copper has an average atomic mass of 63.55 amu and two naturally Copper Has An Average Atomic Mass Of 63.55 U From the above data, the. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. As you know, the average atomic mass of an. Copper Has An Average Atomic Mass Of 63.55 U.
From www.toppr.com
Assume that each atom of copper contributes one electron. The density Copper Has An Average Atomic Mass Of 63.55 U The relat abundance and atomic masses are 69.2% for a. As you know, the average atomic mass of an element is determined. You can approach this problem by using a single. A naturally occuring sample of copper consists of. Let the percentage of.63 29 c u isotope = x. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65. Copper Has An Average Atomic Mass Of 63.55 U.
From scoop.eduncle.com
Ee copper has a mass density p =8.95 gm/cm and an electrical Copper Has An Average Atomic Mass Of 63.55 U The relat abundance and atomic masses are 69.2% for a. Let the percentage of.63 29 c u isotope = x. You can approach this problem by using a single. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u.. Copper Has An Average Atomic Mass Of 63.55 U.
From www.dreamstime.com
Copper Atom, with Mass and Energy Levels. Stock Vector Illustration Copper Has An Average Atomic Mass Of 63.55 U Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. You can approach this problem by using a single. ∴ the percentage of.65 29 c u isotope = 100 − x. As you know, the average atomic mass. Copper Has An Average Atomic Mass Of 63.55 U.
From www.youtube.com
Copper has the fcc crystal structure . Assuming an atomic radius of 130 Copper Has An Average Atomic Mass Of 63.55 U A naturally occuring sample of copper consists of. As you know, the average atomic mass of an element is determined. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and. Copper Has An Average Atomic Mass Of 63.55 U.
From present5.com
Isotopes Atoms of the same element with Copper Has An Average Atomic Mass Of 63.55 U 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. ∴ the percentage of.65 29 c u isotope = 100 − x. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. You can approach this problem by using a single.. Copper Has An Average Atomic Mass Of 63.55 U.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Copper Has An Average Atomic Mass Of 63.55 U The element copper has naturally occurring isotopes with mass numbers of 63 and 65. From the above data, the. A naturally occuring sample of copper consists of. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The. Copper Has An Average Atomic Mass Of 63.55 U.
From lessonfullflotillas.z21.web.core.windows.net
How To Average Atomic Mass Copper Has An Average Atomic Mass Of 63.55 U You can approach this problem by using a single. Let the percentage of.63 29 c u isotope = x. The relat abundance and atomic masses are 69.2% for a. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. As you know, the average atomic mass of an element is determined. ∴ the. Copper Has An Average Atomic Mass Of 63.55 U.
From www.studocu.com
Average Atomic Mass Assignment Average Atomic Mass Daily Check Copper Has An Average Atomic Mass Of 63.55 U If the natural abundance of lighter isotope is 20% than the average atomic mass of the element is: 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. As you know,. Copper Has An Average Atomic Mass Of 63.55 U.
From www.sciencephoto.com
Copper, atomic structure Stock Image C045/6369 Science Photo Library Copper Has An Average Atomic Mass Of 63.55 U From the above data, the. As you know, the average atomic mass of an element is determined. ∴ the percentage of.65 29 c u isotope = 100 − x. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu). Copper Has An Average Atomic Mass Of 63.55 U.
From periodictableguide.com
Copper (Cu) Periodic Table (Element Information & More) Copper Has An Average Atomic Mass Of 63.55 U The relat abundance and atomic masses are 69.2% for a. Let the percentage of.63 29 c u isotope = x. From the above data, the. As you know, the average atomic mass of an element is determined. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. You can approach this problem by using a single.. Copper Has An Average Atomic Mass Of 63.55 U.
From www.alamy.com
Copper atom hires stock photography and images Alamy Copper Has An Average Atomic Mass Of 63.55 U A naturally occuring sample of copper consists of. The relat abundance and atomic masses are 69.2% for a. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. The element copper has naturally occurring isotopes with mass numbers. Copper Has An Average Atomic Mass Of 63.55 U.
From www.numerade.com
⏩SOLVEDCalculate Copper has two isotopes C u63 (abundance =69.2 Copper Has An Average Atomic Mass Of 63.55 U If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. From the above data, the. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. A naturally occuring sample of copper consists of. You can approach this problem by using a. Copper Has An Average Atomic Mass Of 63.55 U.
From www.toppr.com
The average atomic mass of copper is 63.546 amu. Natural copper Copper Has An Average Atomic Mass Of 63.55 U The relat abundance and atomic masses are 69.2% for a. ∴ the percentage of.65 29 c u isotope = 100 − x. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928. Copper Has An Average Atomic Mass Of 63.55 U.
From www.sliderbase.com
Isotopes Copper Has An Average Atomic Mass Of 63.55 U The element copper has naturally occurring isotopes with mass numbers of 63 and 65. Let the percentage of.63 29 c u isotope = x. A naturally occuring sample of copper consists of. From the above data, the. As you know, the average atomic mass of an element is determined. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65. Copper Has An Average Atomic Mass Of 63.55 U.
From www.numerade.com
SOLVEDNaturally occurring copper, Cu, is composed of two isotopes. The Copper Has An Average Atomic Mass Of 63.55 U The relat abundance and atomic masses are 69.2% for a. If we look at the periodic table, copper metal (a mixture of isotopes but 63cu and 65cu predominate) has an approximate atomic. A naturally occuring sample of copper consists of. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. Let the percentage of.63 29 c. Copper Has An Average Atomic Mass Of 63.55 U.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Copper Has An Average Atomic Mass Of 63.55 U A naturally occuring sample of copper consists of. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Let the percentage. Copper Has An Average Atomic Mass Of 63.55 U.
From www.numerade.com
SOLVEDWhat is the mass in grams of one copper atom? Copper Has An Average Atomic Mass Of 63.55 U The element copper has naturally occurring isotopes with mass numbers of 63 and 65. You can approach this problem by using a single. From the above data, the. 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. ∴ the percentage of.65 29 c u isotope = 100 − x. Let the percentage. Copper Has An Average Atomic Mass Of 63.55 U.
From brainly.com
Copper has two isotopes. Copper63, which has an atomic mass of of 62. Copper Has An Average Atomic Mass Of 63.55 U As you know, the average atomic mass of an element is determined. Let the percentage of.63 29 c u isotope = x. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. ∴ the percentage of.65 29 c. Copper Has An Average Atomic Mass Of 63.55 U.
From scoop.eduncle.com
Copper has fcc structure and the atomic radius is 1.278 a.u. calculate Copper Has An Average Atomic Mass Of 63.55 U 69% for the isotope that weighs 62.93 u and 31% for the isotope that weighs 64.93 u. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. From the above data, the. ∴ the percentage of.65 29 c u isotope = 100 − x. If the natural abundance of lighter isotope is 20% than the average. Copper Has An Average Atomic Mass Of 63.55 U.