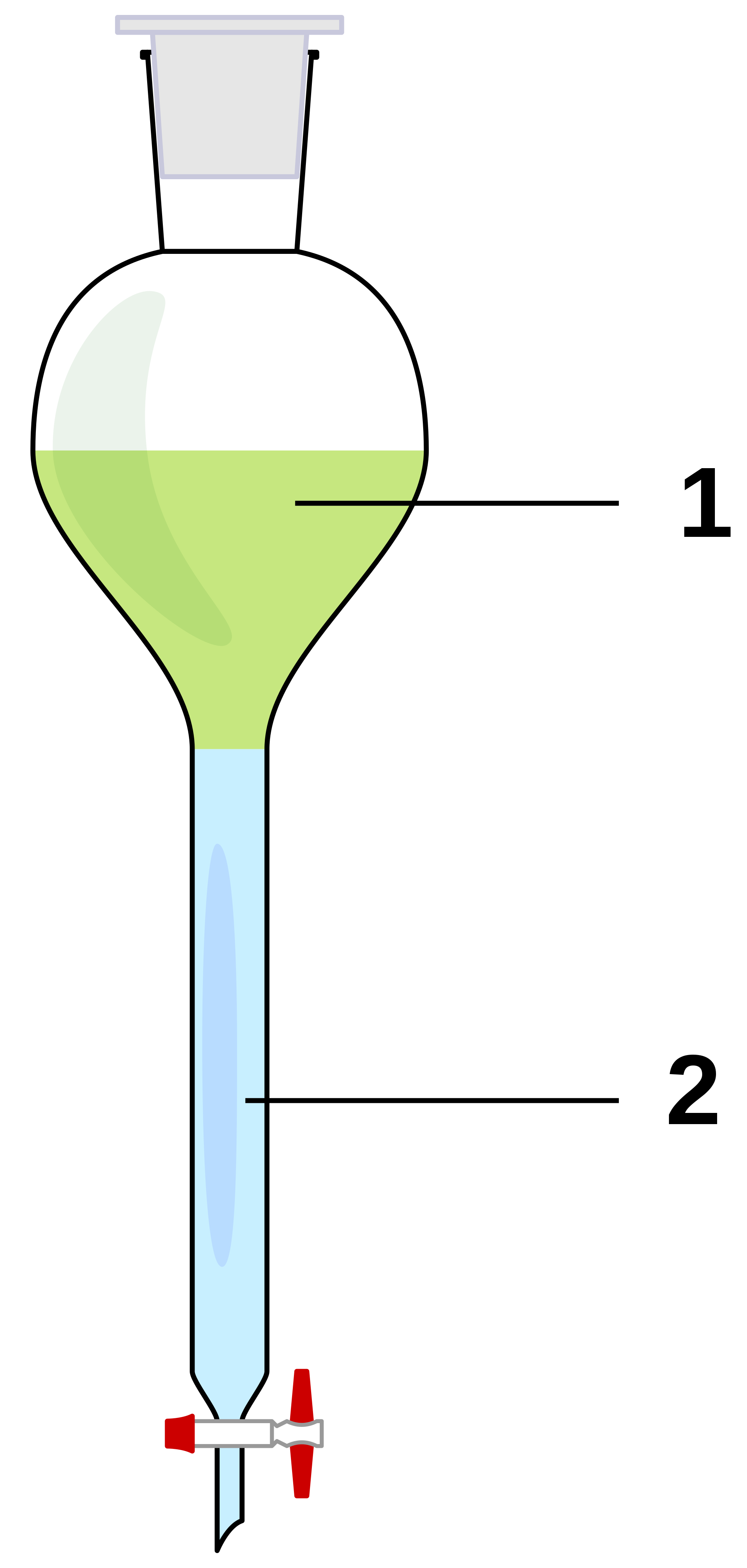
Separating Funnel A Level Chemistry . Higher density liquid (typically aqueous) is the bottom layer, and organic. Put a stopper in the funnel and,. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. The separating funnel works by allowing the two immiscible liquids to separate into two layers. To separate into two layers: how does separating funnel work? Put a stopper in the funnel and, making sure the tap at the bottom is. Intro to general chemistry 3h 48m. revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Transfer the contents of the flask to separating funnel. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Allow the layers to separate and discard the lower (aqueous) la. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution.
from ncvs2.books.nba.co.za
Allow the layers to separate and discard the lower (aqueous) la. how does separating funnel work? Put a stopper in the funnel and, making sure the tap at the bottom is. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. To separate into two layers: Higher density liquid (typically aqueous) is the bottom layer, and organic. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Put a stopper in the funnel and,. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Transfer the contents of the flask to separating funnel.
Unit 2 Separating mixtures National Curriculum (Vocational) Physical
Separating Funnel A Level Chemistry The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Put a stopper in the funnel and,. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Put a stopper in the funnel and, making sure the tap at the bottom is. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Transfer the contents of the flask to separating funnel. Allow the layers to separate and discard the lower (aqueous) la. Higher density liquid (typically aqueous) is the bottom layer, and organic. The separating funnel works by allowing the two immiscible liquids to separate into two layers. To separate into two layers: Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Intro to general chemistry 3h 48m. how does separating funnel work?
From ncvs2.books.nba.co.za
Unit 2 Separating mixtures National Curriculum (Vocational) Physical Separating Funnel A Level Chemistry The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Higher density liquid (typically aqueous) is the bottom layer, and organic. Intro to general chemistry 3h 48m. Pour the distillate into the separating. Separating Funnel A Level Chemistry.
From www.sciencephoto.com
Using a separating funnel Stock Image H460/0486 Science Photo Library Separating Funnel A Level Chemistry Put a stopper in the funnel and,. Higher density liquid (typically aqueous) is the bottom layer, and organic. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Transfer the contents of the. Separating Funnel A Level Chemistry.
From school.careers360.com
methods of separation Overview, Structure, Properties & Uses Separating Funnel A Level Chemistry To separate into two layers: Intro to general chemistry 3h 48m. Put a stopper in the funnel and,. Put a stopper in the funnel and, making sure the tap at the bottom is. Allow the layers to separate and discard the lower (aqueous) la. The separating funnel works by allowing the two immiscible liquids to separate into two layers. . Separating Funnel A Level Chemistry.
From mavink.com
Separating Funnel Diagram Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Transfer the contents of the flask to separating funnel. The separating funnel works by allowing the two immiscible liquids to separate into two layers. how does separating funnel work? To separate into two layers: Pour the distillate into the separating. Separating Funnel A Level Chemistry.
From www.geeksforgeeks.org
Methods of Separation Various Separation Techniques Separating Funnel A Level Chemistry Put a stopper in the funnel and,. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Put a stopper in the funnel and, making sure the tap at the bottom is. Higher density liquid (typically aqueous) is the bottom layer, and organic. how does separating funnel work? The separating funnel works by. Separating Funnel A Level Chemistry.
From www.sciencelabsupplies.in
Pear Shaped Separating Funnels Separating Funnels, Glassware Separating Funnel A Level Chemistry Intro to general chemistry 3h 48m. The separating funnel works by allowing the two immiscible liquids to separate into two layers. Allow the layers to separate and discard the lower (aqueous) la. revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Pour the distillate into the separating funnel and add. Separating Funnel A Level Chemistry.
From eduinput.com
Separatory FunnelPrinciple, Parts, Types, Sizes, and Applications Separating Funnel A Level Chemistry To separate into two layers: how does separating funnel work? Put a stopper in the funnel and,. Put a stopper in the funnel and, making sure the tap at the bottom is. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Intro to general chemistry 3h 48m.. Separating Funnel A Level Chemistry.
From www.youtube.com
How to separate immiscible liquids Separating Funnel Separation Separating Funnel A Level Chemistry Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Put a stopper in the funnel and, making sure the tap at the bottom is. Higher density liquid (typically aqueous) is the bottom layer, and organic. To separate into two layers: Pour the distillate into the separating funnel and add an equal volume of. Separating Funnel A Level Chemistry.
From www.thesciencehive.co.uk
Organic Synthesis* — the science sauce Separating Funnel A Level Chemistry how does separating funnel work? Put a stopper in the funnel and,. Transfer the contents of the flask to separating funnel. Allow the layers to separate and discard the lower (aqueous) la. The separating funnel works by allowing the two immiscible liquids to separate into two layers. Higher density liquid (typically aqueous) is the bottom layer, and organic. The. Separating Funnel A Level Chemistry.
From themasterchemistry.com
Separating FunnelWorking Principle And Uses Separating Funnel A Level Chemistry how does separating funnel work? Intro to general chemistry 3h 48m. Higher density liquid (typically aqueous) is the bottom layer, and organic. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top.. Separating Funnel A Level Chemistry.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separating Funnel A Level Chemistry The separating funnel works by allowing the two immiscible liquids to separate into two layers. how does separating funnel work? Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Higher density liquid (typically aqueous) is the. Separating Funnel A Level Chemistry.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separating Funnel A Level Chemistry Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Put a stopper in the funnel and, making sure the tap at the bottom is. revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus,. Separating Funnel A Level Chemistry.
From themasterchemistry.com
Separating FunnelWorking Principle And Uses Separating Funnel A Level Chemistry Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Transfer the contents of the flask to separating funnel. how does separating funnel work? revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus,. Separating Funnel A Level Chemistry.
From stock.adobe.com
separating funnel diagram. Scientific vector illustration isolated on Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. To separate into two layers: The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. how does separating funnel work? Put a stopper in the funnel and, making sure. Separating Funnel A Level Chemistry.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separating Funnel A Level Chemistry Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Put a stopper in the funnel and,. The separating funnel works by allowing the two immiscible liquids to separate into two layers. Transfer the contents of the flask to separating funnel. Higher density liquid (typically aqueous) is the bottom layer, and organic. Pour the. Separating Funnel A Level Chemistry.
From www.geeksforgeeks.org
Methods of Separation Various Separation Techniques Separating Funnel A Level Chemistry Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Transfer the contents of the flask to separating funnel. Put a stopper in the funnel and, making sure the tap at the bottom is. how does separating funnel work? To separate into two layers: The separating funnel works by allowing the two immiscible. Separating Funnel A Level Chemistry.
From www.youtube.com
Separating Funnel Model ThinkTac DIY Science YouTube Separating Funnel A Level Chemistry Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Transfer the contents of the flask to separating funnel. Allow the layers to separate and discard the lower (aqueous) la. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Put a stopper. Separating Funnel A Level Chemistry.
From www.kartelllabware.com
Graduated Separating Funnel Graduated And Volumetric Plasticware Separating Funnel A Level Chemistry Transfer the contents of the flask to separating funnel. revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Allow the layers to separate and discard the lower (aqueous) la. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top.. Separating Funnel A Level Chemistry.
From www.dreamstime.com
Conical Separating Funnel with Solution Stock Vector Illustration of Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Allow the layers to separate and discard the lower (aqueous) la. The separating funnel works by allowing the two immiscible liquids to separate into two layers. how does separating funnel work? The denser liquid will sink to the bottom of. Separating Funnel A Level Chemistry.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separating Funnel A Level Chemistry The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Higher density liquid (typically aqueous) is the bottom layer, and organic. To separate into two layers: Put a stopper in the funnel and, making sure the tap at the bottom is. The separating funnel works by allowing the two. Separating Funnel A Level Chemistry.
From brainly.in
neat labeled diagram of separating funnel Brainly.in Separating Funnel A Level Chemistry The separating funnel works by allowing the two immiscible liquids to separate into two layers. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Transfer the contents of the flask to separating funnel. Intro to general chemistry 3h 48m. how does separating funnel work? Put a stopper. Separating Funnel A Level Chemistry.
From eduinput.com
Separatory FunnelPrinciple, Parts, Types, Sizes, and Applications Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Allow the layers to. Separating Funnel A Level Chemistry.
From ssh-gulf.com
Separating Funnel, BroadShape Scientific Supply House Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Put a stopper in the funnel and, making sure the tap at the bottom is. To separate into two layers: Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Pour the distillate into the. Separating Funnel A Level Chemistry.
From www.kofastudy.com
Separating Funnel Kofa Study Separating Funnel A Level Chemistry The separating funnel works by allowing the two immiscible liquids to separate into two layers. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. how does separating funnel work? Transfer the contents of the flask to separating funnel. Put a stopper in the funnel and,. To separate into two layers: Higher density. Separating Funnel A Level Chemistry.
From dxoosvtyj.blob.core.windows.net
Separating Funnel Scientific Diagram at Linda Dyer blog Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Put a stopper in the funnel and, making sure the tap at the bottom is. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. The denser liquid will sink to the bottom of the. Separating Funnel A Level Chemistry.
From www.youtube.com
ALevel PreLab Video for Using a Separating Funnel YouTube Separating Funnel A Level Chemistry Put a stopper in the funnel and, making sure the tap at the bottom is. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. revision notes on 4.6.1 techniques for the. Separating Funnel A Level Chemistry.
From www.vecteezy.com
separating funnel used to seperate two immiscible solvent phases vector Separating Funnel A Level Chemistry Intro to general chemistry 3h 48m. The separating funnel works by allowing the two immiscible liquids to separate into two layers. Transfer the contents of the flask to separating funnel. The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. To separate into two layers: revision notes on. Separating Funnel A Level Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记7.6.5 Organic Techniques Purification Separating Funnel A Level Chemistry The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. The separating funnel works by allowing the two immiscible liquids to separate into two layers. Intro to general chemistry 3h 48m. Transfer the contents of the flask to separating funnel. Pour the distillate into the separating funnel and add. Separating Funnel A Level Chemistry.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. how does separating funnel work? Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Put a stopper in the funnel and, making sure the tap at the bottom is. Intro to general chemistry. Separating Funnel A Level Chemistry.
From www.tradeindia.com
Borosilicate Laboratory Separating Funnel at Best Price in Ambala S D Separating Funnel A Level Chemistry Put a stopper in the funnel and,. Transfer the contents of the flask to separating funnel. The separating funnel works by allowing the two immiscible liquids to separate into two layers. Intro to general chemistry 3h 48m. To separate into two layers: Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Allow the. Separating Funnel A Level Chemistry.
From www.shutterstock.com
Separating Funnel Diagram Scientific Vector Illustration Stock Vector Separating Funnel A Level Chemistry revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Higher density liquid (typically aqueous) is the bottom layer, and organic. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Allow the layers to separate and discard the lower (aqueous) la. Put a stopper. Separating Funnel A Level Chemistry.
From www.vecteezy.com
lab glass Separation funnel with PTFE Stopper diagram for experiment Separating Funnel A Level Chemistry The separating funnel works by allowing the two immiscible liquids to separate into two layers. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Intro to general chemistry 3h 48m. Put a stopper in the funnel and,. Separating Funnel A Level Chemistry.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separating Funnel A Level Chemistry Intro to general chemistry 3h 48m. To separate into two layers: The denser liquid will sink to the bottom of the funnel, and the less dense liquid will float to the top. Put a stopper in the funnel and, making sure the tap at the bottom is. how does separating funnel work? Allow the layers to separate and discard. Separating Funnel A Level Chemistry.
From www.slideshare.net
Separating funnel Separating Funnel A Level Chemistry Higher density liquid (typically aqueous) is the bottom layer, and organic. how does separating funnel work? revision notes on 4.6.1 techniques for the ocr a level chemistry syllabus, written by the chemistry experts at. Transfer the contents of the flask to separating funnel. The separating funnel works by allowing the two immiscible liquids to separate into two layers.. Separating Funnel A Level Chemistry.
From glyndwrebeny.blogspot.com
12+ diagram of separating funnel GlyndwrEbeny Separating Funnel A Level Chemistry how does separating funnel work? To separate into two layers: Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. Allow the layers to separate and discard the lower (aqueous) la. Pour the distillate into the separating funnel and add an equal volume of saturated nacl solution. The denser liquid will sink to. Separating Funnel A Level Chemistry.