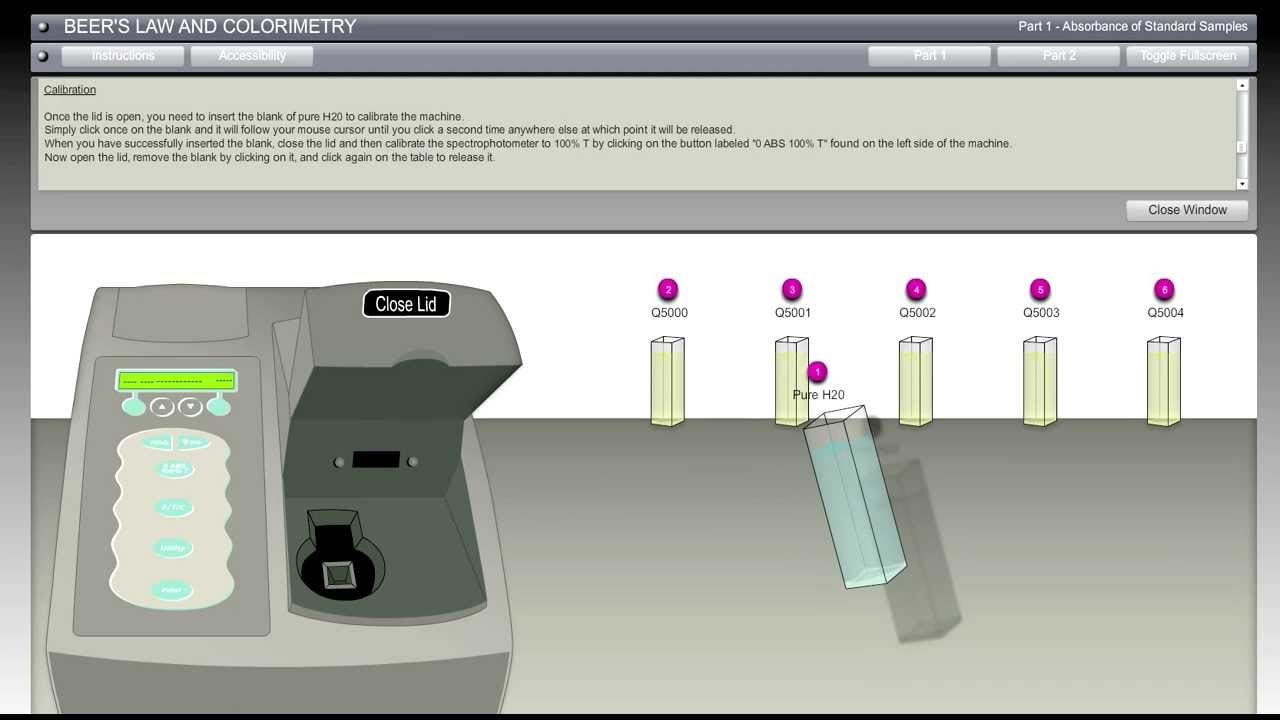
Spectrophotometer Beer's Law . make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! beer's law states that a chemical solution's concentration is directly proportional to its light absorption. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer’s law indicates that there is a linear relationship between absorbance and concentration. The premise is that a light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.
from www.youtube.com
since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The premise is that a light. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer’s law indicates that there is a linear relationship between absorbance and concentration. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that a chemical solution's concentration is directly proportional to its light absorption.
Spectrophotometer Beer's Law And Colorimetry Lab YouTube
Spectrophotometer Beer's Law beer's law states that a chemical solution's concentration is directly proportional to its light absorption. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The premise is that a light. beer’s law indicates that there is a linear relationship between absorbance and concentration. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer!
From www.thoughtco.com
Beer's Law Definition and Equation Spectrophotometer Beer's Law in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law indicates that there is a linear relationship between absorbance and concentration. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Spectrophotometer Beer's Law.
From www.youtube.com
BeerLambert's law with derivation/ Absorbance & Transmittance Spectrophotometer Beer's Law make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. The premise is that a light. beer’s law indicates that there is a linear relationship between absorbance. Spectrophotometer Beer's Law.
From www.youtube.com
How to compute for the concentration of the unknown? Spectrophotometer Spectrophotometer Beer's Law The premise is that a light. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! beer's law states that a chemical solution's concentration is directly proportional to its light absorption. beer’s law indicates that there is a linear relationship between absorbance and concentration. in spectroscopy, beer’s. Spectrophotometer Beer's Law.
From icuvets.com
UV Visible Spectrophotometer Setup And BeerLambert Law ICuvets Cells Spectrophotometer Beer's Law in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The premise is that a light. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer's law states that a chemical. Spectrophotometer Beer's Law.
From gionlecoh.blob.core.windows.net
Beer Lambert Law Wavelength at Viola Hitt blog Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer’s law indicates that there is a linear relationship between absorbance and concentration. The premise is that a light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Spectrophotometer Beer's Law.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Spectrophotometer Beer's Law beer’s law indicates that there is a linear relationship between absorbance and concentration. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! . Spectrophotometer Beer's Law.
From study.com
BeerLambert Law Equation, Units & Examples Lesson Spectrophotometer Beer's Law The premise is that a light. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer’s law indicates that there is a linear relationship between absorbance and concentration. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to. Spectrophotometer Beer's Law.
From sciencenotes.org
Beer's Law Equation and Example Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit. Spectrophotometer Beer's Law.
From mungfali.com
UV Spectroscopy Beer Lambert Law Spectrophotometer Beer's Law The premise is that a light. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law indicates that there is a linear relationship. Spectrophotometer Beer's Law.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Spectrophotometer Beer's Law beer’s law indicates that there is a linear relationship between absorbance and concentration. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. The premise is that. Spectrophotometer Beer's Law.
From glossary.periodni.com
Beer’s law Chemistry Dictionary & Glossary Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The premise is that a light. since the concentration, path length and molar absorptivity are all directly. Spectrophotometer Beer's Law.
From www.youtube.com
B.Sc 3rd year zoology Spectrophotometer Beerlambert Law Light Spectrophotometer Beer's Law in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The premise is that a light. the amount of light that a. Spectrophotometer Beer's Law.
From hxewwgvce.blob.core.windows.net
Beer Lambert Law With Equation at Erma Walden blog Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. The premise is that a light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. make colorful concentrated and dilute solutions. Spectrophotometer Beer's Law.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation, free Spectrophotometer Beer's Law since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The premise is that a light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. the amount of light that a. Spectrophotometer Beer's Law.
From www.youtube.com
Spectrophotometer Beer's Law And Colorimetry Lab YouTube Spectrophotometer Beer's Law in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The premise is that a light. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. the amount of light that a species absorbs in a spectroscopic transition. Spectrophotometer Beer's Law.
From www.chegg.com
SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. The premise is that a light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that a chemical. Spectrophotometer Beer's Law.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its. Spectrophotometer Beer's Law.
From www.chegg.com
Solved SPECTROPHOTOMETRY. APPLY BEER'S LAW TO DETERMINE Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! beer’s law indicates that there is a linear relationship between absorbance and concentration. The premise is that. Spectrophotometer Beer's Law.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. The premise is that a light. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. beer’s law indicates that there is a linear relationship between absorbance and concentration. in. Spectrophotometer Beer's Law.
From mungfali.com
UV Spectroscopy Beer Lambert Law Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light. beer’s law indicates that there is a linear relationship between absorbance and concentration. make. Spectrophotometer Beer's Law.
From www.slideserve.com
PPT Determining An Equilibrium Constant Using Spectrophotometry and Spectrophotometer Beer's Law The premise is that a light. beer’s law indicates that there is a linear relationship between absorbance and concentration. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in. Spectrophotometer Beer's Law.
From www.youtube.com
UV VIS Spectroscopy 02 Spectrophotometer Beer Lambert Law Spectrophotometer Beer's Law since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light. the amount of light that a species absorbs in a spectroscopic transition can be related. Spectrophotometer Beer's Law.
From ecency.com
Spectrophotometry Simplified The BeerLambert Law in Spectrophotom... Spectrophotometer Beer's Law make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law indicates that there is a linear relationship between absorbance and concentration. . Spectrophotometer Beer's Law.
From www.youtube.com
Weight Percent Calculation from direct Reading (ppm) of UVVis Spectrophotometer Beer's Law make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! beer’s law indicates that there is a linear relationship between absorbance and concentration. The premise is that a light. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number. Spectrophotometer Beer's Law.
From slidetodoc.com
Beers Lambert Law and Standard Curves BCH 312 Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. The premise is that a light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law indicates that there is. Spectrophotometer Beer's Law.
From www.youtube.com
Beer Lambert Law Demonstration Tamil Colorimeter Spectrophotometer Beer's Law since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. beer’s law indicates that there is a linear relationship between absorbance and concentration. the amount of light that a species. Spectrophotometer Beer's Law.
From www.chegg.com
Solved SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Spectrophotometer Beer's Law the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Spectrophotometer Beer's Law.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Spectrophotometer Beer's Law make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The premise is that a light. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to. Spectrophotometer Beer's Law.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Spectrophotometer Beer's Law beer’s law indicates that there is a linear relationship between absorbance and concentration. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The premise is that. Spectrophotometer Beer's Law.
From www.studocu.com
Chem 201 Experiment 1 Introduction to Spectrophotometer & Beer's Law Spectrophotometer Beer's Law in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law indicates that there is a linear relationship between absorbance and. Spectrophotometer Beer's Law.
From www.youtube.com
Spectrophotometer UVVIS Spectrophotometer BeerLambert Law Uttam Spectrophotometer Beer's Law beer's law states that a chemical solution's concentration is directly proportional to its light absorption. beer’s law indicates that there is a linear relationship between absorbance and concentration. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. the amount of light. Spectrophotometer Beer's Law.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Spectrophotometer Beer's Law The premise is that a light. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer’s law indicates that there is a linear relationship between absorbance and concentration. in. Spectrophotometer Beer's Law.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Spectrophotometer Beer's Law since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law indicates that there is a linear relationship between absorbance and concentration. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The premise is that. Spectrophotometer Beer's Law.
From www.youtube.com
Spectrophotometer and Beer's Law YouTube Spectrophotometer Beer's Law The premise is that a light. beer’s law indicates that there is a linear relationship between absorbance and concentration. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in. Spectrophotometer Beer's Law.
From stock.adobe.com
schematic diagram of spectrophotometer, UV visible spectrophotometer Spectrophotometer Beer's Law The premise is that a light. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! since the concentration, path length and molar absorptivity. Spectrophotometer Beer's Law.