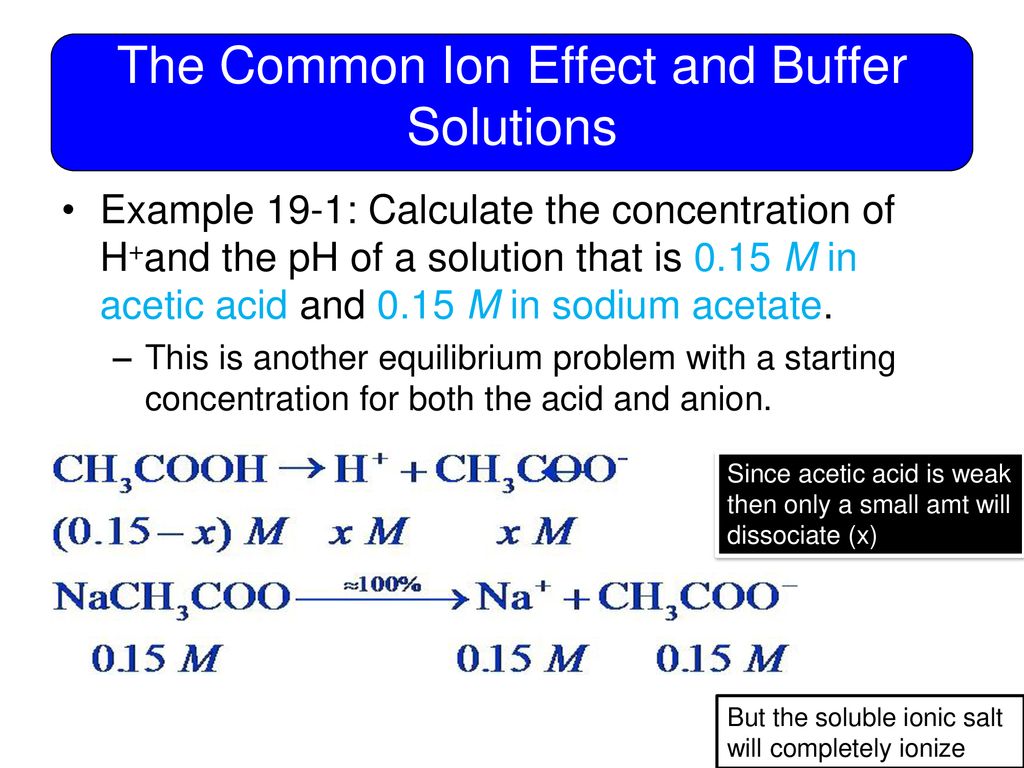
Common Ion Buffer Solution . a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. Either weak acids and their.
from slideplayer.com
adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant.
Ionic Equilibria Part II Buffers and Titration Curves ppt download
Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Common Ion Buffer Solution Either weak acids and their. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion. Common Ion Buffer Solution.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Common Ion Buffer Solution Either weak acids and their. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion. Common Ion Buffer Solution.
From www.studypool.com
SOLUTION Lecture 10 buffer solutions titrations solubility and common Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium. Common Ion Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Buffer Solution a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion. Common Ion Buffer Solution.
From www.scribd.com
6 Buffers, Common Ion and HH PDF Buffer Solution Acid Common Ion Buffer Solution Either weak acids and their. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect. Common Ion Buffer Solution.
From www.researchgate.net
Scheme of the ion's dissociation and hydration in buffer solution with Common Ion Buffer Solution Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion. Common Ion Buffer Solution.
From www.scribd.com
Common Ion Effect and Buffer Solution PDF Buffer Solution Acid Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Either weak acids and their. adding a common ion. Common Ion Buffer Solution.
From oneclass.com
OneClass 55 of 57 Common ions Effect A buffer solution is 0.24 M NH3 Common Ion Buffer Solution Either weak acids and their. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion. Common Ion Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Buffer Solution a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. the common ion effect. Common Ion Buffer Solution.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not. Common Ion Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. a buffer solution is a solution. Common Ion Buffer Solution.
From www.scribd.com
Common Ion Effect and Buffer Solution PDF Buffer Solution Acid Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not. Common Ion Buffer Solution.
From www.slideserve.com
PPT Chapter 16 Introduction to Buffers PowerPoint Presentation, free Common Ion Buffer Solution Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion. Common Ion Buffer Solution.
From www.pinterest.com
Common Ion Effect Buffer Solution and Solubility Product Solubility Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not. Common Ion Buffer Solution.
From www.studypool.com
SOLUTION Common Ion Effect and Buffer Solution Presentation Studypool Common Ion Buffer Solution a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium. Common Ion Buffer Solution.
From www.scribd.com
Cape Chemistry Buffers and Titration Common Ion PDF Buffer Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not. Common Ion Buffer Solution.
From www.studypool.com
SOLUTION Lecture 10 buffer solutions titrations solubility and common Common Ion Buffer Solution a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. Either weak acids and their. adding a common ion. Common Ion Buffer Solution.
From www.studypool.com
SOLUTION Ionic equilibrium note 7 buffer solution salt hydrolysis and Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. Either weak acids and their. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution. Common Ion Buffer Solution.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Either weak acids and their. the common ion effect. Common Ion Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Buffer Solution Either weak acids and their. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution. Common Ion Buffer Solution.
From www.youtube.com
Common ions effect, buffers solutions and Acid base titration for Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution. Common Ion Buffer Solution.
From www.studocu.com
Lecture 14 Common ion effect and Buffer Solutions Common ion effect Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium. Common Ion Buffer Solution.
From www.slideserve.com
PPT Ionic Equilibria 離子平衡 Part II Buffers 緩衝液 and Titration Curves Common Ion Buffer Solution Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution. Common Ion Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Common Ion Buffer Solution a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. the common ion effect. Common Ion Buffer Solution.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. Either weak acids and their. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion. Common Ion Buffer Solution.
From www.youtube.com
Common Ion Effect, Buffers Solutions and Change in pH Part 4 YouTube Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution where the ph does not. Common Ion Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Buffer Solution a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. the common ion effect describes the effect on equilibrium. Common Ion Buffer Solution.
From www.youtube.com
Common Ion Effect and Buffer Solutions YouTube Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium. Common Ion Buffer Solution.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution. Common Ion Buffer Solution.
From wordmint.com
common ion effect and buffer solution Word Scramble WordMint Common Ion Buffer Solution adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the common ion effect describes the effect on equilibrium. Common Ion Buffer Solution.
From www.studypool.com
SOLUTION Analytical chemistry common ion effect buffer solution notes Common Ion Buffer Solution Either weak acids and their. the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. a buffer solution is a solution. Common Ion Buffer Solution.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. a buffer solution is a solution. Common Ion Buffer Solution.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or. Common Ion Buffer Solution.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. adding a common ion prevents the weak acid or. Common Ion Buffer Solution.
From www.youtube.com
The Common Ion Effect and Buffer Solutions YouTube Common Ion Buffer Solution the common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in the. adding a common ion prevents the weak acid or weak base from ionizing as much as it would without the added common ion. Either weak acids and their. a buffer solution is a solution. Common Ion Buffer Solution.