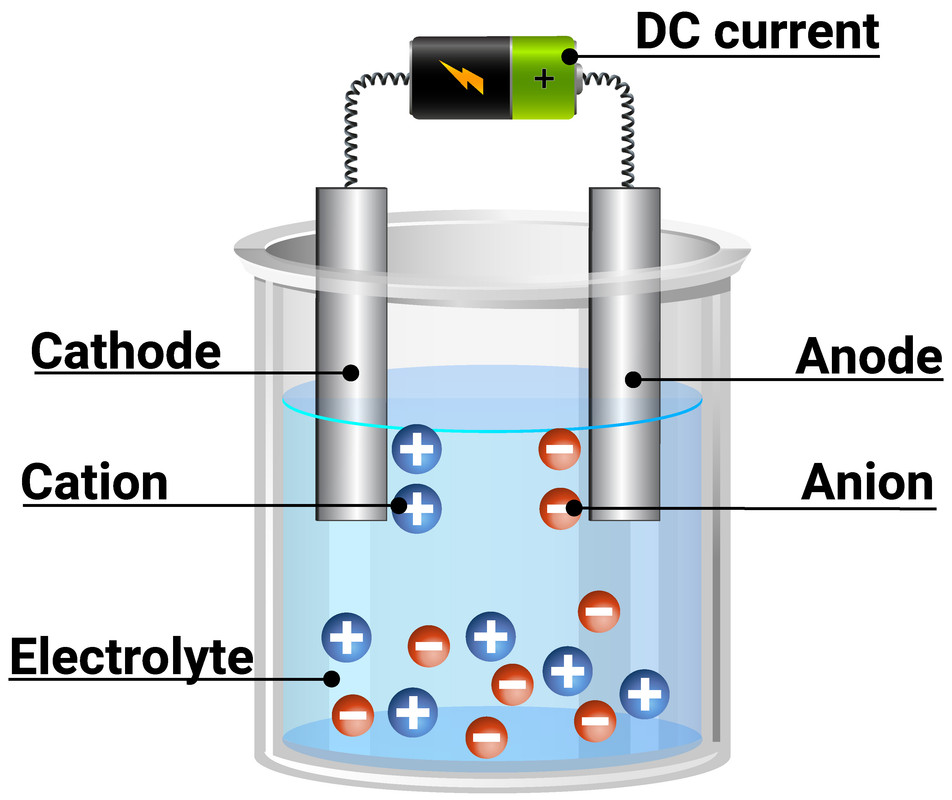
Electrodes In Electrolysis That Don't React . the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. Find out the definitions and. learn how electrolysis is used to extract reactive metals from their ores using an electric current. The electrodes that do not react are called inert electrodes or auxiliary electrodes. learn how electrolysis separates ionic substances by passing an electric current through them. Electrolysis can also be used to. See the equations for electrolysis of water, sodium chloride, and. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. See examples of electrolysis of. Learn more about electrolysis, its applications, and how to calculate the cell potential.
from studycopesettic.z21.web.core.windows.net
Electrolysis can also be used to. Learn more about electrolysis, its applications, and how to calculate the cell potential. learn how electrolysis separates ionic substances by passing an electric current through them. See the equations for electrolysis of water, sodium chloride, and. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. learn how electrolysis is used to extract reactive metals from their ores using an electric current. See examples of electrolysis of. The electrodes that do not react are called inert electrodes or auxiliary electrodes.
Inert Electrodes Gcse
Electrodes In Electrolysis That Don't React learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. The electrodes that do not react are called inert electrodes or auxiliary electrodes. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. learn how electrolysis is used to extract reactive metals from their ores using an electric current. Find out the definitions and. Learn more about electrolysis, its applications, and how to calculate the cell potential. Electrolysis can also be used to. See examples of electrolysis of. learn how electrolysis separates ionic substances by passing an electric current through them. See the equations for electrolysis of water, sodium chloride, and.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Electrodes In Electrolysis That Don't React See examples of electrolysis of. Electrolysis can also be used to. learn how electrolysis is used to extract reactive metals from their ores using an electric current. See the equations for electrolysis of water, sodium chloride, and. Find out the definitions and. learn how electrolysis separates ionic substances by passing an electric current through them. in electrolysis,. Electrodes In Electrolysis That Don't React.
From studyrocket.co.uk
Electrolysis GCSE Chemistry Science) OCR Revision Study Electrodes In Electrolysis That Don't React the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. The electrodes that do not react are. Electrodes In Electrolysis That Don't React.
From www.adevos.science
Electrolysis Adevoscience Electrodes In Electrolysis That Don't React in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. See examples of electrolysis of. Find out the definitions and. learn how electrolysis is used to extract reactive metals from their ores using. Electrodes In Electrolysis That Don't React.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Electrodes In Electrolysis That Don't React learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. See the equations for electrolysis of water, sodium chloride, and. The electrodes that do not react are called inert electrodes or auxiliary electrodes. Learn more about electrolysis, its applications, and how to calculate the cell potential. learn how electrolysis separates ionic substances. Electrodes In Electrolysis That Don't React.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrodes In Electrolysis That Don't React Learn more about electrolysis, its applications, and how to calculate the cell potential. See the equations for electrolysis of water, sodium chloride, and. See examples of electrolysis of. Find out the definitions and. The electrodes that do not react are called inert electrodes or auxiliary electrodes. the potential applied to the electrode pair is what enables the above reaction. Electrodes In Electrolysis That Don't React.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes In Electrolysis That Don't React learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. learn how electrolysis separates ionic substances by passing an electric current through them. The electrodes that do not react are called inert electrodes or auxiliary electrodes. Learn more about electrolysis, its applications, and how to calculate the cell potential. See the equations. Electrodes In Electrolysis That Don't React.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 Electrodes In Electrolysis That Don't React in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. See the equations for electrolysis of water, sodium chloride, and. Find out the definitions and. learn how electrolysis is used to extract reactive metals from their ores using an electric current. The electrodes that do not react are called inert electrodes or auxiliary electrodes. learn. Electrodes In Electrolysis That Don't React.
From pubs.acs.org
Accurate Potentials of Hg/HgO Electrodes Practical Parameters for Electrodes In Electrolysis That Don't React Electrolysis can also be used to. learn how electrolysis is used to extract reactive metals from their ores using an electric current. Find out the definitions and. See the equations for electrolysis of water, sodium chloride, and. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at. Electrodes In Electrolysis That Don't React.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Electrodes In Electrolysis That Don't React in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. See the equations for electrolysis of water, sodium chloride, and. See examples of electrolysis of. Find out the definitions and. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how inert. Electrodes In Electrolysis That Don't React.
From schematicodtautobuses5n.z4.web.core.windows.net
Draw A Labelled Diagram Of Electrolytic Cell Electrodes In Electrolysis That Don't React Find out the definitions and. Learn more about electrolysis, its applications, and how to calculate the cell potential. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. See the equations for electrolysis of. Electrodes In Electrolysis That Don't React.
From www.knowledgeboat.com
Chapter 6 Electrolysis Selina Solutions Concise Chemistry Class 10 Electrodes In Electrolysis That Don't React learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. Learn more about electrolysis, its applications, and how to calculate the cell potential. The electrodes that do not react are called inert electrodes or auxiliary electrodes. learn how electrolysis separates ionic substances by passing an electric current through them. See examples of. Electrodes In Electrolysis That Don't React.
From philschatz.com
Electrolytes · Chemistry Electrodes In Electrolysis That Don't React See examples of electrolysis of. Learn more about electrolysis, its applications, and how to calculate the cell potential. Electrolysis can also be used to. learn how electrolysis is used to extract reactive metals from their ores using an electric current. See the equations for electrolysis of water, sodium chloride, and. Find out the definitions and. in electrolysis, an. Electrodes In Electrolysis That Don't React.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Electrodes In Electrolysis That Don't React See the equations for electrolysis of water, sodium chloride, and. Learn more about electrolysis, its applications, and how to calculate the cell potential. learn how electrolysis is used to extract reactive metals from their ores using an electric current. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction. Electrodes In Electrolysis That Don't React.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrodes In Electrolysis That Don't React Learn more about electrolysis, its applications, and how to calculate the cell potential. See the equations for electrolysis of water, sodium chloride, and. See examples of electrolysis of. Find out the definitions and. learn how electrolysis is used to extract reactive metals from their ores using an electric current. learn how inert electrodes do not react with the. Electrodes In Electrolysis That Don't React.
From ceujucww.blob.core.windows.net
Inert Electrodes In Electrolysis at Isidro Holland blog Electrodes In Electrolysis That Don't React learn how electrolysis separates ionic substances by passing an electric current through them. See the equations for electrolysis of water, sodium chloride, and. learn how electrolysis is used to extract reactive metals from their ores using an electric current. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. learn how inert electrodes do. Electrodes In Electrolysis That Don't React.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrodes In Electrolysis That Don't React Find out the definitions and. Learn more about electrolysis, its applications, and how to calculate the cell potential. The electrodes that do not react are called inert electrodes or auxiliary electrodes. See the equations for electrolysis of water, sodium chloride, and. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. the. Electrodes In Electrolysis That Don't React.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Electrodes In Electrolysis That Don't React learn how electrolysis separates ionic substances by passing an electric current through them. Find out the definitions and. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to. The electrodes that do not react are called inert electrodes or auxiliary electrodes. See the equations for electrolysis of water, sodium chloride,. Electrodes In Electrolysis That Don't React.
From chemistry.stackexchange.com
electrochemistry Standard electrode potential for electrolytic vs Electrodes In Electrolysis That Don't React See the equations for electrolysis of water, sodium chloride, and. Learn more about electrolysis, its applications, and how to calculate the cell potential. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how electrolysis is used to extract reactive metals from their ores using. Electrodes In Electrolysis That Don't React.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrodes In Electrolysis That Don't React The electrodes that do not react are called inert electrodes or auxiliary electrodes. learn how electrolysis is used to extract reactive metals from their ores using an electric current. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how inert electrodes do not. Electrodes In Electrolysis That Don't React.
From www.youtube.com
what is the meaning of electrolysis chemistry electrodes Electrodes In Electrolysis That Don't React Find out the definitions and. Electrolysis can also be used to. See examples of electrolysis of. learn how electrolysis is used to extract reactive metals from their ores using an electric current. learn how electrolysis separates ionic substances by passing an electric current through them. Learn more about electrolysis, its applications, and how to calculate the cell potential.. Electrodes In Electrolysis That Don't React.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Electrodes In Electrolysis That Don't React Find out the definitions and. Electrolysis can also be used to. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. See examples of electrolysis of. Learn more about electrolysis, its applications, and how to calculate the cell potential. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. The. Electrodes In Electrolysis That Don't React.
From www.snexplores.org
Explainer What is an electrode? Electrodes In Electrolysis That Don't React Electrolysis can also be used to. learn how electrolysis separates ionic substances by passing an electric current through them. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how electrolysis is used to extract reactive metals from their ores using an electric current.. Electrodes In Electrolysis That Don't React.
From www.researchgate.net
Scheme of twoelectrode system for overall water splitting. HER Electrodes In Electrolysis That Don't React See examples of electrolysis of. Electrolysis can also be used to. See the equations for electrolysis of water, sodium chloride, and. The electrodes that do not react are called inert electrodes or auxiliary electrodes. learn how electrolysis separates ionic substances by passing an electric current through them. learn how inert electrodes do not react with the electrolyte or. Electrodes In Electrolysis That Don't React.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry Electrodes In Electrolysis That Don't React Learn more about electrolysis, its applications, and how to calculate the cell potential. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. See the equations for electrolysis of water, sodium chloride, and. Electrolysis can also be used to. learn how electrolysis is used to extract. Electrodes In Electrolysis That Don't React.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki Electrodes In Electrolysis That Don't React Electrolysis can also be used to. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. Find out the definitions and. learn how electrolysis is used to extract reactive metals from their ores using an electric current. learn. Electrodes In Electrolysis That Don't React.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process Electrodes In Electrolysis That Don't React Find out the definitions and. See the equations for electrolysis of water, sodium chloride, and. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. Electrolysis can also be used to. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the potential applied to the electrode pair is. Electrodes In Electrolysis That Don't React.
From www.youtube.com
AQA Required Practical The electrolysis of copper (II) chloride Electrodes In Electrolysis That Don't React The electrodes that do not react are called inert electrodes or auxiliary electrodes. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. See the equations for electrolysis of water, sodium chloride, and. Learn more about electrolysis, its applications, and how to calculate the cell potential. learn how electrolysis separates ionic substances. Electrodes In Electrolysis That Don't React.
From library.fiveable.me
Electrolysis Intro to Chemistry Study Guide 2024 Fiveable Electrodes In Electrolysis That Don't React learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. learn how electrolysis is used to extract reactive metals from their ores using an electric current. learn how electrolysis separates ionic substances by passing an electric current through them. See examples of electrolysis of. the potential applied to the electrode. Electrodes In Electrolysis That Don't React.
From philschatz.com
Electrolysis · Chemistry Electrodes In Electrolysis That Don't React See the equations for electrolysis of water, sodium chloride, and. Learn more about electrolysis, its applications, and how to calculate the cell potential. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. learn how electrolysis separates ionic substances. Electrodes In Electrolysis That Don't React.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrodes In Electrolysis That Don't React See the equations for electrolysis of water, sodium chloride, and. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. Find out the definitions and. Electrolysis can also be used to. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the potential applied to the electrode pair is. Electrodes In Electrolysis That Don't React.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Electrodes In Electrolysis That Don't React the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. Electrolysis can also be used to. learn how electrolysis is used to extract reactive metals from their ores using an electric current. See the equations for electrolysis of water, sodium chloride, and. The electrodes that do. Electrodes In Electrolysis That Don't React.
From www.dreamstime.com
Electrolysis of Water Splitting H2O Molecules Stock Illustration Electrodes In Electrolysis That Don't React Electrolysis can also be used to. See the equations for electrolysis of water, sodium chloride, and. Find out the definitions and. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. Learn more about electrolysis, its applications, and how to calculate the cell potential. learn how. Electrodes In Electrolysis That Don't React.
From owlcation.com
Electrolysis The Way of the Future Owlcation Electrodes In Electrolysis That Don't React in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. Learn more about electrolysis, its applications, and how to calculate the cell potential. The electrodes that do not react are called inert electrodes or auxiliary electrodes. See the equations for. Electrodes In Electrolysis That Don't React.
From www.chemicals.co.uk
What is a Chemical Change? The Chemistry Blog Electrodes In Electrolysis That Don't React in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. The electrodes that do not react are called inert electrodes or auxiliary electrodes. Electrolysis can also be used to. Find out the definitions and.. Electrodes In Electrolysis That Don't React.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Electrodes In Electrolysis That Don't React Find out the definitions and. the potential applied to the electrode pair is what enables the above reaction to occur without a paired reduction reaction at the. learn how inert electrodes do not react with the electrolyte or the products formed during electrolysis. learn how electrolysis is used to extract reactive metals from their ores using an. Electrodes In Electrolysis That Don't React.