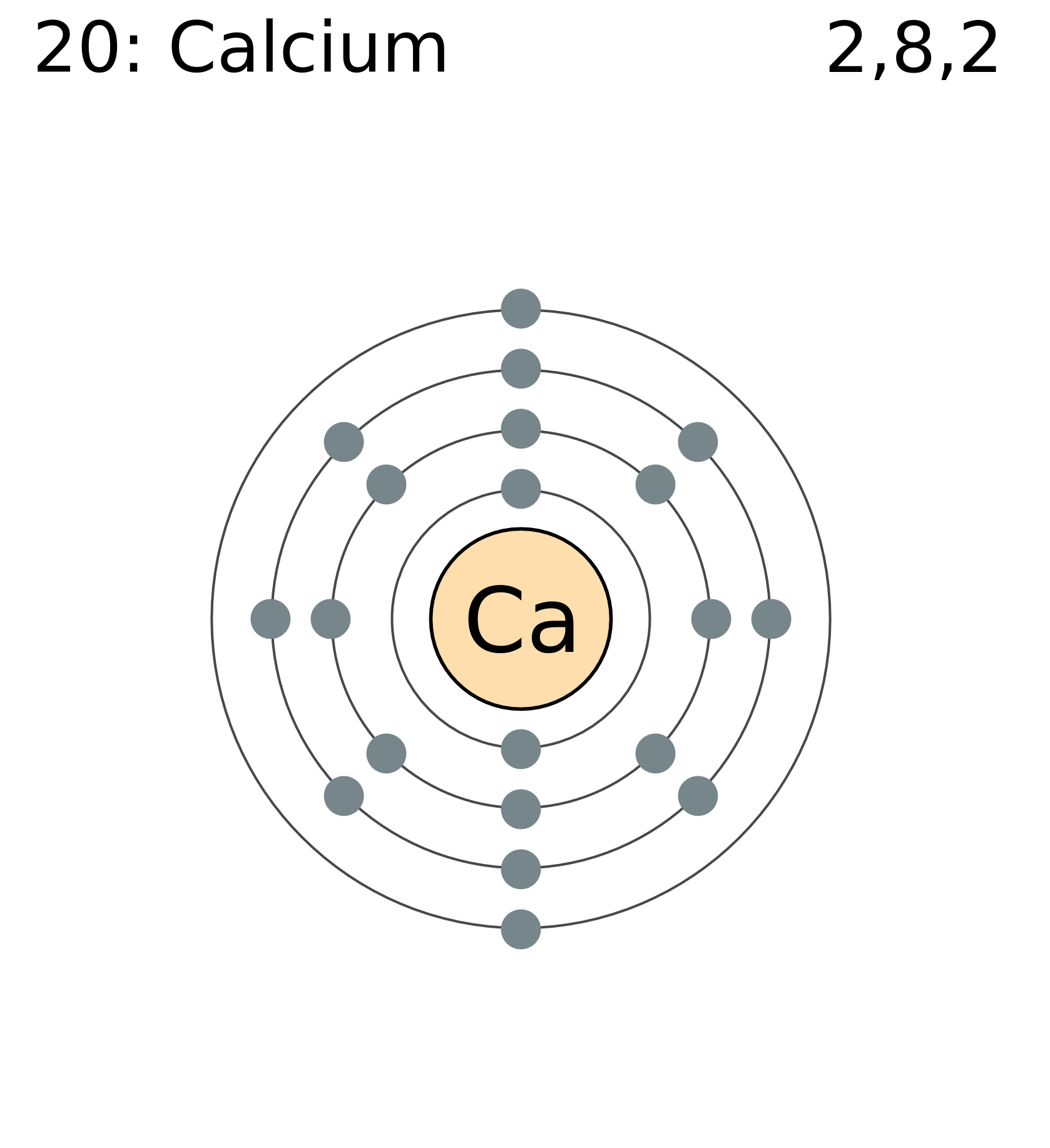
Electron Shell Configuration Calcium . It determines bonding and reactivity. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. The next electron is added to complete the 4s. The calcium ion (ca 2+), however, has two. electron configuration helps predict how atoms will interact. hence, potassium corresponds to li and na in its valence shell configuration. hence, potassium corresponds to li and na in its valence shell configuration. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. The next electron is added to complete the. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 119 rows electron configuration chart of all elements is mentioned in the table below.
from commons.wikimedia.org
hence, potassium corresponds to li and na in its valence shell configuration. electron configuration helps predict how atoms will interact. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. The next electron is added to complete the. The calcium ion (ca 2+), however, has two. hence, potassium corresponds to li and na in its valence shell configuration. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. It determines bonding and reactivity. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. The next electron is added to complete the 4s.
FileElectron shell 020 calcium.png
Electron Shell Configuration Calcium The next electron is added to complete the 4s. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. electron configuration helps predict how atoms will interact. hence, potassium corresponds to li and na in its valence shell configuration. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. The next electron is added to complete the. 119 rows electron configuration chart of all elements is mentioned in the table below. The calcium ion (ca 2+), however, has two. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. hence, potassium corresponds to li and na in its valence shell configuration. It determines bonding and reactivity. The next electron is added to complete the 4s. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20.
From imgbin.com
Electron Shell Calcium Electron Configuration Valence Electron PNG Electron Shell Configuration Calcium for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The calcium ion (ca 2+), however, has two. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. while writing. Electron Shell Configuration Calcium.
From www.alamy.com
Calcium (Ca). Diagram of the nuclear composition and electron Electron Shell Configuration Calcium while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. The next electron is added to complete the 4s. The next electron is added to complete the. hence, potassium corresponds to li and na in its valence shell configuration. in order to write the calcium electron configuration we first need to. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Calcium Electron Configuration Arrows Electron Shell Configuration Calcium for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. electron configuration helps predict how atoms will interact. It determines bonding and reactivity. 119 rows electron configuration chart of all elements is mentioned. Electron Shell Configuration Calcium.
From material-properties.org
Calcium Protons Neutrons Electrons Electron Configuration Electron Shell Configuration Calcium hence, potassium corresponds to li and na in its valence shell configuration. The calcium ion (ca 2+), however, has two. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. electron configuration helps predict how atoms will interact. 119 rows electron configuration chart of all elements is mentioned in the. Electron Shell Configuration Calcium.
From www.sciencephoto.com
Calcium electron configuration Stock Image C029/5027 Science Electron Shell Configuration Calcium in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. The next electron is added to complete the 4s. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. It determines bonding and reactivity. The next electron is added to complete the. hence,. Electron Shell Configuration Calcium.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Electron Shell Configuration Calcium hence, potassium corresponds to li and na in its valence shell configuration. hence, potassium corresponds to li and na in its valence shell configuration. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The next electron is added to complete the. So ca. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Electron Shell Model Of Calcium Electron Shell Configuration Calcium electron configuration helps predict how atoms will interact. 119 rows electron configuration chart of all elements is mentioned in the table below. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. It determines bonding and reactivity. hence, potassium corresponds to li and na in its valence shell configuration. . Electron Shell Configuration Calcium.
From mydiagram.online
[DIAGRAM] Atomic Diagram For Calcium Electron Shell Configuration Calcium The next electron is added to complete the. The next electron is added to complete the 4s. 119 rows electron configuration chart of all elements is mentioned in the table below. electron configuration helps predict how atoms will interact. hence, potassium corresponds to li and na in its valence shell configuration. hence, potassium corresponds to li. Electron Shell Configuration Calcium.
From sciencenotes.org
Calcium Facts Electron Shell Configuration Calcium for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. It determines bonding and reactivity. electron configuration helps predict how atoms will interact. The next electron is added to complete the 4s. hence, potassium corresponds to li and na in its valence shell configuration.. Electron Shell Configuration Calcium.
From www.mozaweb.com
Electron configuration of calcium 3D scene Mozaik Digital Learning Electron Shell Configuration Calcium while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. electron configuration helps predict how atoms will interact. The calcium ion (ca 2+), however, has two. hence,. Electron Shell Configuration Calcium.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Calcium In Ground State worksheet Electron Shell Configuration Calcium It determines bonding and reactivity. hence, potassium corresponds to li and na in its valence shell configuration. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. hence, potassium corresponds to li and na in its valence shell configuration. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6. Electron Shell Configuration Calcium.
From www.alamy.com
Periodic Table of the Elements, Shell Structure of Calcium Ca Electron Shell Configuration Calcium It determines bonding and reactivity. The calcium ion (ca 2+), however, has two. The next electron is added to complete the. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. hence, potassium corresponds to li and na in its valence shell configuration. electron configuration helps predict how atoms will interact.. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Calcium Electron Configuration Arrows Electron Shell Configuration Calcium in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. 119 rows electron configuration chart of all elements is mentioned in the table below. hence, potassium corresponds to li and na in its valence shell configuration. So ca electron configuration is 1s2 2s2. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Electron Shell Diagram Calcium Electron Shell Configuration Calcium The next electron is added to complete the 4s. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. hence, potassium corresponds to li and na in its valence shell configuration. The next electron is added to complete the. for instance, the ground. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Electron Shell Model Of Calcium Electron Shell Configuration Calcium It determines bonding and reactivity. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. hence, potassium corresponds to li and na in its valence shell configuration. The next electron is added to complete the. electron configuration helps predict how atoms will interact. The. Electron Shell Configuration Calcium.
From periodictable.me
Calcium Electron Configuration (Ca) with Orbital Diagram Electron Shell Configuration Calcium So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. The next electron is added to complete the. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 119 rows electron configuration chart of all elements is mentioned in the table below. electron. Electron Shell Configuration Calcium.
From iperiodictable.com
Calcium Electron Configuration Periodic Table Electron Shell Configuration Calcium The next electron is added to complete the. The next electron is added to complete the 4s. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. while writing the configuration we put. Electron Shell Configuration Calcium.
From topblogtenz.com
Calcium Orbital diagram, Electron configuration, and Valence electrons Electron Shell Configuration Calcium electron configuration helps predict how atoms will interact. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. 119 rows electron configuration chart of all elements. Electron Shell Configuration Calcium.
From periodictable.me
Calcium Electron Configuration (Ca) with Orbital Diagram Electron Shell Configuration Calcium The next electron is added to complete the. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 119 rows electron configuration chart of all elements is mentioned. Electron Shell Configuration Calcium.
From proper-cooking.info
Electron Shell Model Of Calcium Electron Shell Configuration Calcium The next electron is added to complete the 4s. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 119 rows electron configuration chart of all elements is mentioned in the table below. in order to write the calcium electron configuration we first need. Electron Shell Configuration Calcium.
From valenceelectrons.com
How Many Valence Electrons Does Calcium (Ca) Have? Electron Shell Configuration Calcium while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. hence, potassium corresponds to li and na in its valence shell configuration. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. electron configuration helps predict how. Electron Shell Configuration Calcium.
From www.webelements.com
Elements Periodic Table » Calcium » properties of free atoms Electron Shell Configuration Calcium hence, potassium corresponds to li and na in its valence shell configuration. It determines bonding and reactivity. The next electron is added to complete the. while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. 119 rows electron configuration chart of all elements is mentioned in the table below. electron. Electron Shell Configuration Calcium.
From www.newtondesk.com
Calcium Ca (Element 20) of Periodic Table Elements FlashCards Electron Shell Configuration Calcium The next electron is added to complete the. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. It determines bonding and reactivity. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. hence, potassium corresponds to li and na in its valence shell. Electron Shell Configuration Calcium.
From commons.wikimedia.org
FileElectron shell 020 calcium.png Electron Shell Configuration Calcium in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. The calcium ion (ca 2+), however, has two. electron configuration helps predict how atoms will interact. The next electron is added to complete the. for instance, the ground state electronic configuration of calcium. Electron Shell Configuration Calcium.
From www.pinterest.com
Calcium Art Print by Carlos Clarivan Atom, Electron configuration Electron Shell Configuration Calcium So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. It determines bonding and reactivity. hence, potassium corresponds to li and na in its valence shell configuration. for instance, the ground state. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Electron Shell Diagram Calcium Electron Shell Configuration Calcium So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. hence, potassium corresponds to li and na in its valence shell configuration. The next electron is added to complete the 4s. The calcium ion (ca 2+), however, has two. in order to write the calcium electron configuration we first need to know the number of electrons for. Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
3d Bohr Model Of Calcium Electron Shell Configuration Calcium 119 rows electron configuration chart of all elements is mentioned in the table below. The next electron is added to complete the 4s. The next electron is added to complete the. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. So ca electron configuration. Electron Shell Configuration Calcium.
From worksheetsday.blogspot.com
Electron Configuration Of Calcium Ca Worksheets Electron Shell Configuration Calcium while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. It determines bonding and reactivity. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. 119 rows electron configuration chart of all elements is mentioned in the. Electron Shell Configuration Calcium.
From animalia-life.club
Electron Configuration For Calcium Electron Shell Configuration Calcium The next electron is added to complete the. 119 rows electron configuration chart of all elements is mentioned in the table below. It determines bonding and reactivity. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. hence, potassium corresponds to li and na. Electron Shell Configuration Calcium.
From schematicliblatinas77.z22.web.core.windows.net
Electron Dot Diagram Calcium Electron Shell Configuration Calcium while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. The next electron is added to complete the 4s. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. The calcium ion (ca 2+), however, has two. . Electron Shell Configuration Calcium.
From ar.inspiredpencil.com
Electron Shell Diagram Calcium Electron Shell Configuration Calcium The next electron is added to complete the. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. electron configuration helps predict how atoms will interact. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. in order to write the calcium electron. Electron Shell Configuration Calcium.
From sarahminglenn.blogspot.com
Electron Arrangement of Calcium SarahminGlenn Electron Shell Configuration Calcium It determines bonding and reactivity. hence, potassium corresponds to li and na in its valence shell configuration. The calcium ion (ca 2+), however, has two. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. for instance, the ground state electronic configuration of. Electron Shell Configuration Calcium.
From www.animalia-life.club
Electron Shell Diagram Electron Shell Configuration Calcium while writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. in order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20. hence, potassium corresponds to li and na in its valence shell configuration. The next electron is added. Electron Shell Configuration Calcium.
From animalia-life.club
Electron Configuration For Calcium Electron Shell Configuration Calcium So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. 119 rows electron configuration chart of all elements is mentioned in the table below. It determines bonding and reactivity. The calcium ion (ca 2+), however, has two. in order to write the calcium electron configuration we first need to know the number of electrons for the ca. Electron Shell Configuration Calcium.
From periodictable.me
Calcium Electron Configuration (Ca) with Orbital Diagram Electron Shell Configuration Calcium It determines bonding and reactivity. for instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. So ca electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2. hence, potassium corresponds to li and na in its valence shell configuration. electron configuration helps predict how atoms. Electron Shell Configuration Calcium.