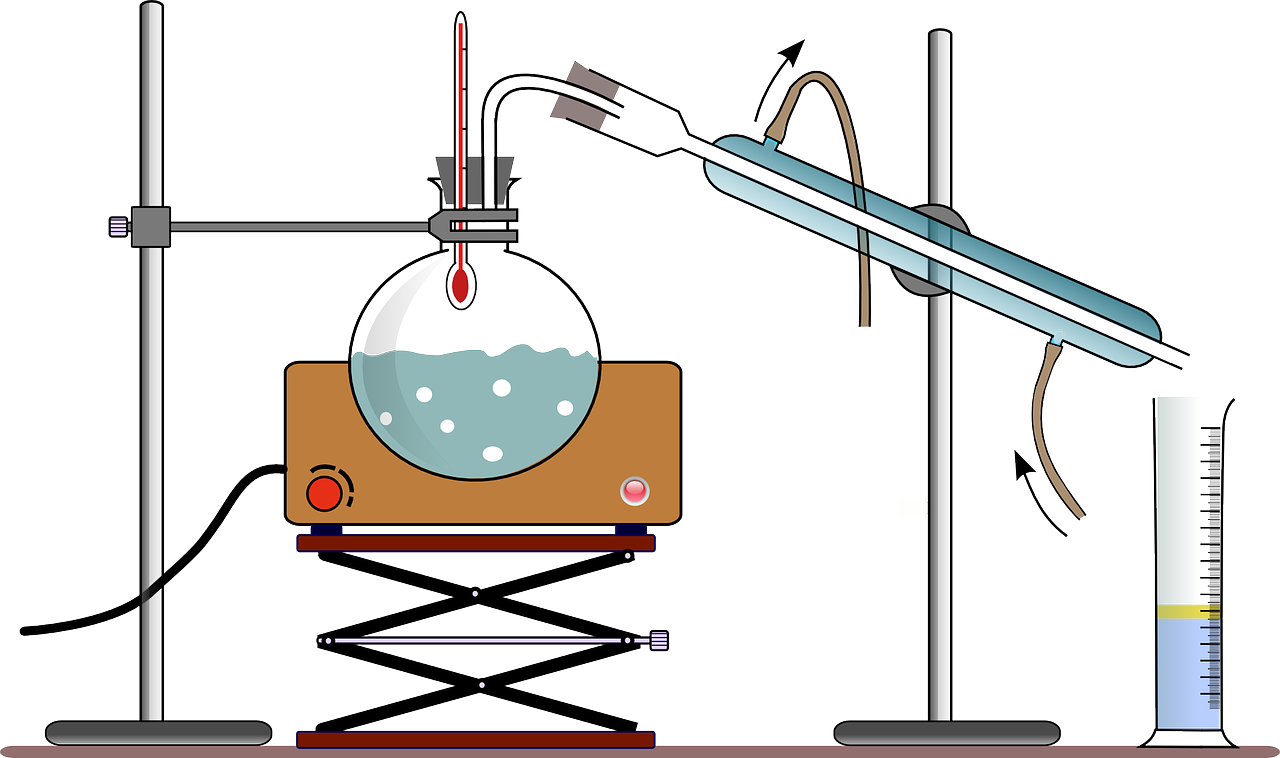
Filtration And Distillation Take Advantage Of . Filtration separates solids of different sizes. The principle of filtration is to separate the solids. the easiest method to separate a liquid from an insoluble solid is filtration. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). distillation takes advantage of differences in boiling points. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. Evaporation removes a liquid from a solution to leave a solid material. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the. Particle size can vary considerably, given the type of mixture. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. Distillation (as discussed in analysis:. In this blog post, we will explore three commonly used separation methods:
from www.waterpronto.com
for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. the easiest method to separate a liquid from an insoluble solid is filtration. from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. Particle size can vary considerably, given the type of mixture. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. In this blog post, we will explore three commonly used separation methods: The principle of filtration is to separate the solids. Filtration separates solids of different sizes. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation).
Water Purification By Distillation Process Explained
Filtration And Distillation Take Advantage Of the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. distillation takes advantage of differences in boiling points. the easiest method to separate a liquid from an insoluble solid is filtration. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. In this blog post, we will explore three commonly used separation methods: Distillation (as discussed in analysis:. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). The principle of filtration is to separate the solids. Filtration separates solids of different sizes. from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. Particle size can vary considerably, given the type of mixture. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. Evaporation removes a liquid from a solution to leave a solid material. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final.
From www.freepik.com
Premium AI Image Mineral Water Distillation and Filtration Filtration And Distillation Take Advantage Of 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). distillation takes advantage of differences in boiling points. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. Distillation (as discussed in analysis:. In this blog post, we will explore three commonly. Filtration And Distillation Take Advantage Of.
From dxondimvq.blob.core.windows.net
Filtration And Distillation Method at Mary Bowser blog Filtration And Distillation Take Advantage Of Evaporation removes a liquid from a solution to leave a solid material. the easiest method to separate a liquid from an insoluble solid is filtration. The principle of filtration is to separate the solids. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a. Filtration And Distillation Take Advantage Of.
From www.bbc.co.uk
Distillation BBC Bitesize Filtration And Distillation Take Advantage Of Filtration separates solids of different sizes. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Evaporation removes a liquid from a solution to leave a solid material. Particle size can vary considerably, given the type of mixture. for example, chromatography is a separation process based on solubility 1 and distillation. Filtration And Distillation Take Advantage Of.
From dxondimvq.blob.core.windows.net
Filtration And Distillation Method at Mary Bowser blog Filtration And Distillation Take Advantage Of 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). The principle of filtration is to separate the solids. Particle size can vary considerably, given the type of mixture. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough. Filtration And Distillation Take Advantage Of.
From www.dreamstime.com
Water Purification Process with Filtration and Distillation in Filtration And Distillation Take Advantage Of filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. Particle size can vary considerably, given the. Filtration And Distillation Take Advantage Of.
From www.etsy.com
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy Filtration And Distillation Take Advantage Of for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. In this blog post, we will explore three commonly used separation methods: filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with. Filtration And Distillation Take Advantage Of.
From www.slideserve.com
PPT Simple Distillation PowerPoint Presentation, free download ID Filtration And Distillation Take Advantage Of In this blog post, we will explore three commonly used separation methods: distillation takes advantage of differences in boiling points. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. for example, chromatography is a separation. Filtration And Distillation Take Advantage Of.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the. distillation takes advantage of differences in boiling points. Filtration separates solids of different sizes. . Filtration And Distillation Take Advantage Of.
From www.waterpronto.com
Water Purification By Distillation Process Explained Filtration And Distillation Take Advantage Of Evaporation removes a liquid from a solution to leave a solid material. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. the easiest method to separate a liquid from an insoluble solid is filtration. distillation. Filtration And Distillation Take Advantage Of.
From slideplayer.com
Chapter 3 Matter Properties and Changes ppt download Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point.. Filtration And Distillation Take Advantage Of.
From www.studypool.com
SOLUTION Filtration crystallisation distillation Studypool Filtration And Distillation Take Advantage Of distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the. distillation takes advantage of differences in boiling points. The principle of filtration is to separate the solids. 2 this article looks at separation by. Filtration And Distillation Take Advantage Of.
From www.freepik.com
Premium AI Image Mineral Water Distillation and Filtration Filtration And Distillation Take Advantage Of Filtration separates solids of different sizes. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. distillation takes advantage of differences in boiling points. distillation is the use of heat to separate the components of a. Filtration And Distillation Take Advantage Of.
From aimhigh.space
Distillation and Chromatography AimHigh! Filtration And Distillation Take Advantage Of the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. Distillation (as discussed in analysis:. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing. Filtration And Distillation Take Advantage Of.
From www.cleantechwater.co.in
Pros and Cons of Different Water Filtration Methods Filtration And Distillation Take Advantage Of filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration. Filtration And Distillation Take Advantage Of.
From dxoofswss.blob.core.windows.net
How Does Condenser Work In Distillation at Caroline McLaughlin blog Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. the easiest method to separate a liquid from an insoluble solid is filtration. Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. Distillation (as discussed in analysis:. Particle size can vary considerably, given the type of mixture. In this blog post,. Filtration And Distillation Take Advantage Of.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Filtration And Distillation Take Advantage Of the easiest method to separate a liquid from an insoluble solid is filtration. Filtration separates solids of different sizes. distillation takes advantage of differences in boiling points. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous. Filtration And Distillation Take Advantage Of.
From www.vrogue.co
Investigate How To Use Filtration And Distillation To vrogue.co Filtration And Distillation Take Advantage Of the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). The principle of filtration is to separate the solids. distillation takes advantage of differences in boiling points. Particle size can. Filtration And Distillation Take Advantage Of.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Filtration And Distillation Take Advantage Of Filtration separates solids of different sizes. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. Particle size can vary considerably, given the type of mixture. Evaporation removes a liquid from a solution to leave a solid material. 2 this article looks at separation by particle size (filtration) and removal of. Filtration And Distillation Take Advantage Of.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Filtration And Distillation Take Advantage Of Evaporation removes a liquid from a solution to leave a solid material. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. Filtration separates solids of different sizes. distillation takes advantage of differences in boiling points. . Filtration And Distillation Take Advantage Of.
From testbook.com
team Distillation Principle, Working, Advantages & Application Filtration And Distillation Take Advantage Of Particle size can vary considerably, given the type of mixture. Evaporation removes a liquid from a solution to leave a solid material. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Filtration separates solids of different sizes. from purifying water to refining crude oil, these processes are essential for ensuring. Filtration And Distillation Take Advantage Of.
From 12014dorcascharityng.weebly.com
FILTRATION,EVAPORATION,DISTILLATION AND PAPER CHROMATOGRAPHY 3E1 Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. Particle size can vary considerably, given the type of mixture. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough. Filtration And Distillation Take Advantage Of.
From exoibxrus.blob.core.windows.net
Are Filtration And Distillation The Same at Tom Estey blog Filtration And Distillation Take Advantage Of the easiest method to separate a liquid from an insoluble solid is filtration. from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. The principle of filtration is to separate the solids. filtration is a separation method used to separate out pure substances in. Filtration And Distillation Take Advantage Of.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Filtration And Distillation Take Advantage Of In this blog post, we will explore three commonly used separation methods: 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. Particle size can vary considerably, given. Filtration And Distillation Take Advantage Of.
From en.wikipedia.org
Distillation Wikipedia Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. In this blog post, we will explore three commonly used separation methods: from purifying water to refining crude. Filtration And Distillation Take Advantage Of.
From www.askdifference.com
Distillation vs. Filtration — What’s the Difference? Filtration And Distillation Take Advantage Of filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. In this blog post, we will explore three commonly. Filtration And Distillation Take Advantage Of.
From www.dreamstime.com
Water Purification Process with Filtration, Sedimentation and Filtration And Distillation Take Advantage Of from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. the easiest method to separate a liquid from an insoluble solid is filtration. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large. Filtration And Distillation Take Advantage Of.
From www.researchgate.net
Distillation for Water Purification Download Scientific Diagram Filtration And Distillation Take Advantage Of In this blog post, we will explore three commonly used separation methods: 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final. distillation takes advantage of differences in boiling points.. Filtration And Distillation Take Advantage Of.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple Filtration And Distillation Take Advantage Of In this blog post, we will explore three commonly used separation methods: 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or. Filtration And Distillation Take Advantage Of.
From safewaterfilter.in
What Is Distilled Water? Filtration And Distillation Take Advantage Of for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. Evaporation removes a liquid from a solution to leave a solid material. Particle size can vary considerably, given the type of mixture. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of. Filtration And Distillation Take Advantage Of.
From www.thoughtco.com
What Is Distillation? Principles and Uses Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the. the choice between distillation and filtration depends on the nature of the mixture and the. Filtration And Distillation Take Advantage Of.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds Filtration And Distillation Take Advantage Of In this blog post, we will explore three commonly used separation methods: Distillation (as discussed in analysis:. distillation takes advantage of differences in boiling points. from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. the choice between distillation and filtration depends on the. Filtration And Distillation Take Advantage Of.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Filtration And Distillation Take Advantage Of Evaporation removes a liquid from a solution to leave a solid material. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Distillation (as discussed in analysis:. The principle of filtration is to separate the solids. Particle size can vary considerably, given the type of mixture. distillation takes advantage of differences. Filtration And Distillation Take Advantage Of.
From www.freepik.com
Premium AI Image Mineral Water Distillation and Filtration Filtration And Distillation Take Advantage Of The principle of filtration is to separate the solids. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. from purifying water to refining crude oil, these processes are essential for ensuring that the desired substances are obtained with precision and efficiency. Filtration separates solids of different sizes. 2 this. Filtration And Distillation Take Advantage Of.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration And Distillation Take Advantage Of Particle size can vary considerably, given the type of mixture. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration. Filtration And Distillation Take Advantage Of.
From www.studypool.com
SOLUTION Distillation and filtration Studypool Filtration And Distillation Take Advantage Of distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the. filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size. Filtration And Distillation Take Advantage Of.