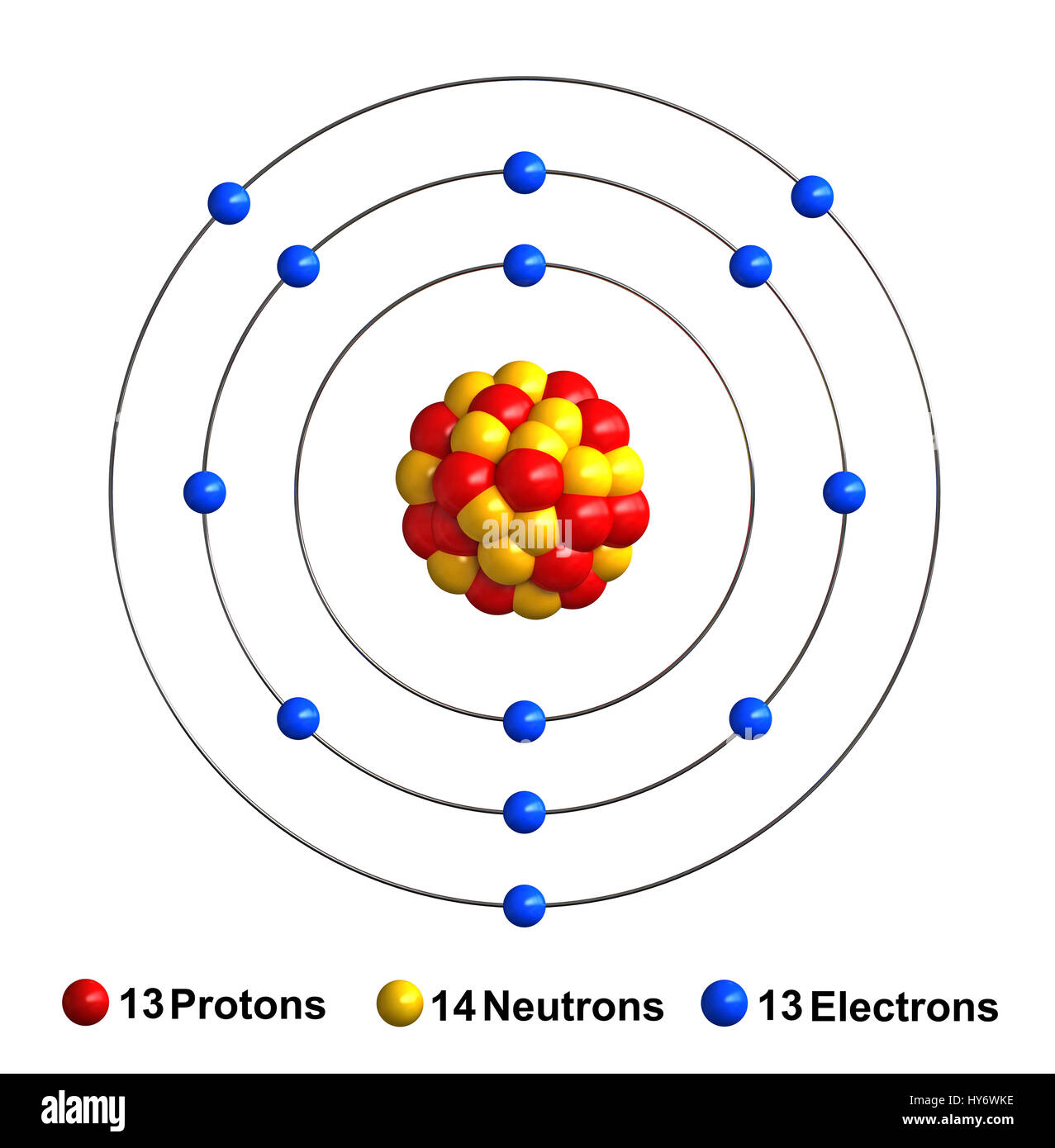
The Aluminum Atom Gains . the aluminum atom loses its three valence electrons. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. The reasons for gaining and losing electrons. Atoms of group 16 gain two electrons and form ions with a 2−. to find the electron configuration of an atom, you first need to know the number of electrons that it has. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Aluminum, for instance, has an atomic number of 13, which. in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. when an atom gains an electron it gains a negative charge and is called an anion. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. atoms of group 17 gain one electron and form anions with a 1− charge; when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.
from www.alamy.com
Aluminum, for instance, has an atomic number of 13, which. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. atoms of group 17 gain one electron and form anions with a 1− charge; in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. to find the electron configuration of an atom, you first need to know the number of electrons that it has. The reasons for gaining and losing electrons. Atoms of group 16 gain two electrons and form ions with a 2−. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.
3d render of atom structure of aluminum isolated over white background
The Aluminum Atom Gains Aluminum, for instance, has an atomic number of 13, which. to find the electron configuration of an atom, you first need to know the number of electrons that it has. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. when an atom gains an electron it gains a negative charge and is called an anion. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. the aluminum atom loses its three valence electrons. The reasons for gaining and losing electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Atoms of group 16 gain two electrons and form ions with a 2−. atoms of group 17 gain one electron and form anions with a 1− charge; Aluminum, for instance, has an atomic number of 13, which. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background The Aluminum Atom Gains Atoms of group 16 gain two electrons and form ions with a 2−. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. atoms of group 17 gain. The Aluminum Atom Gains.
From www.museoinclusivo.com
How Many Electrons Does Aluminum Gain or Lose? Aluminum Profile Blog The Aluminum Atom Gains atoms of group 17 gain one electron and form anions with a 1− charge; The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. Aluminum, for instance, has an atomic number of 13, which. when an atom gains an electron it gains a negative charge. The Aluminum Atom Gains.
From slideplayer.com
Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and ppt The Aluminum Atom Gains when an atom gains an electron it gains a negative charge and is called an anion. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. Aluminum, for instance, has an atomic number of 13, which. atoms of group 17 gain one electron and form. The Aluminum Atom Gains.
From www.numerade.com
SOLVED When aluminum reacts with sulfur to form an ionic compound The Aluminum Atom Gains to find the electron configuration of an atom, you first need to know the number of electrons that it has. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Atoms of group 16 gain two electrons and form ions with a 2−. The reasons for gaining and losing. The Aluminum Atom Gains.
From sciencenotes.org
Aluminum Atom Science Notes and Projects The Aluminum Atom Gains Aluminum, for instance, has an atomic number of 13, which. atoms of group 17 gain one electron and form anions with a 1− charge; The reasons for gaining and losing electrons. Atoms of group 16 gain two electrons and form ions with a 2−. The mg 2 + ion, the al 3 + ion, the na + ion, and. The Aluminum Atom Gains.
From www.numerade.com
SOLVED How many valence electrons does each atom have? Potassium The Aluminum Atom Gains The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Aluminum, for instance, has an atomic number of 13, which. The reasons for gaining and losing electrons.. The Aluminum Atom Gains.
From www.numerade.com
SOLVED When aluminum reacts with sulfur to form an ionic compound The Aluminum Atom Gains in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. Aluminum, for instance, has an atomic number of 13, which. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Atoms of group 16 gain two electrons. The Aluminum Atom Gains.
From tech.noakmech.com
How Many Electrons Does Aluminum Need To Be Stable ZTech The Aluminum Atom Gains when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the aluminum atom loses its three valence electrons. to find the electron configuration of an atom, you first need to know the number of electrons that it has. The reasons for gaining and losing electrons. atoms of. The Aluminum Atom Gains.
From www.numerade.com
SOLVED Select the number of electrons that each atom needs t0 gain The Aluminum Atom Gains in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. atoms of group 17 gain one electron and form anions with a 1− charge; the aluminum atom loses its three valence electrons. For most elements under typical conditions, three electrons is the maximum number that. The Aluminum Atom Gains.
From www.youtube.com
How to Find the Mass of One Atom of Aluminum (Al) YouTube The Aluminum Atom Gains the aluminum atom loses its three valence electrons. Atoms of group 16 gain two electrons and form ions with a 2−. when an atom gains an electron it gains a negative charge and is called an anion. in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13. The Aluminum Atom Gains.
From mavink.com
Aluminum Shell Diagram The Aluminum Atom Gains Aluminum, for instance, has an atomic number of 13, which. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. in the particular example. The Aluminum Atom Gains.
From www.animalia-life.club
Aluminum Bohr Model The Aluminum Atom Gains in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. The mg 2 + ion, the al 3 + ion, the na. The Aluminum Atom Gains.
From www.slideserve.com
PPT Chemistry Unit 6 PowerPoint Presentation, free download ID6652385 The Aluminum Atom Gains For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. when an atom gains an electron it gains a negative charge and is called an anion. to find. The Aluminum Atom Gains.
From www.alamy.com
Aluminium (Al). Diagram of the nuclear composition, electron The Aluminum Atom Gains For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. The reasons for gaining and losing electrons. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. some atoms have nearly eight electrons in their valence shell and. The Aluminum Atom Gains.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma The Aluminum Atom Gains The reasons for gaining and losing electrons. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the aluminum atom loses its three valence electrons. . The Aluminum Atom Gains.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures The Aluminum Atom Gains Aluminum, for instance, has an atomic number of 13, which. The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Atoms of group 16 gain. The Aluminum Atom Gains.
From aluminumgenjin.blogspot.com
Aluminum Aluminum Atom The Aluminum Atom Gains in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. The reasons for gaining and losing electrons. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. when forming ions, elements typically gain or lose the. The Aluminum Atom Gains.
From ar.inspiredpencil.com
Aluminum Atom Diagram The Aluminum Atom Gains The reasons for gaining and losing electrons. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. to find the electron configuration of an atom, you first need. The Aluminum Atom Gains.
From www.nagwa.com
Question Video Determining the Number of Unpaired Electrons in an Atom The Aluminum Atom Gains The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. the aluminum atom loses its three valence electrons. to find the electron configuration of an atom, you first need to know the number of electrons that it has. in certain situations, however, the atom. The Aluminum Atom Gains.
From www.myelectrical2015.com
Electrical Revolution The Aluminum Atom Gains the aluminum atom loses its three valence electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The reasons for gaining and losing electrons. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. when. The Aluminum Atom Gains.
From www.museoinclusivo.com
How Many Aluminum Atoms are Present in Al2O3? Aluminum Profile Blog The Aluminum Atom Gains For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet.. The Aluminum Atom Gains.
From www.dreamstime.com
Aluminum Atom, with Mass and Energy Levels. Stock Vector Illustration The Aluminum Atom Gains when an atom gains an electron it gains a negative charge and is called an anion. the aluminum atom loses its three valence electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Atoms of group 16 gain two electrons and form ions with a 2−. For. The Aluminum Atom Gains.
From www.museoinclusivo.com
Does Aluminum Gain or Lose Electrons? Exploring the Chemical Properties The Aluminum Atom Gains the aluminum atom loses its three valence electrons. in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. some atoms. The Aluminum Atom Gains.
From aluminumgenjin.blogspot.com
Aluminum Aluminum Atom The Aluminum Atom Gains The reasons for gaining and losing electrons. Atoms of group 16 gain two electrons and form ions with a 2−. to find the electron configuration of an atom, you first need to know the number of electrons that it has. when an atom gains an electron it gains a negative charge and is called an anion. Aluminum, for. The Aluminum Atom Gains.
From brainly.in
Atomic structure of aluminum Brainly.in The Aluminum Atom Gains The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. atoms of group 17 gain one electron and form anions with a 1− charge; when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the. The Aluminum Atom Gains.
From rokanggun.blogspot.com
18+ Aluminum Atome The Aluminum Atom Gains The mg 2 + ion, the al 3 + ion, the na + ion, and the elemental n e atom are all isoelectronic. in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. in certain situations, however, the atom may lose or gain one. The Aluminum Atom Gains.
From www.youtube.com
Aluminium atom YouTube The Aluminum Atom Gains in certain situations, however, the atom may lose or gain one or more electrons and acquire a net charge, becoming an ion. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. when an atom gains an electron it gains a negative charge and is called an anion.. The Aluminum Atom Gains.
From www.numerade.com
SOLVED When an aluminum atom (Al) an aluminum ion (Ap+), it The Aluminum Atom Gains when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. to find the electron configuration of an atom, you first need to know the number of electrons that it has. The reasons for gaining and losing electrons. atoms of group 17 gain one electron and form anions with. The Aluminum Atom Gains.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum (Al) Have? The Aluminum Atom Gains For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. atoms of group 17 gain one electron and form anions with a 1− charge; to find the electron configuration of. The Aluminum Atom Gains.
From rokanggun.blogspot.com
18+ Aluminum Atome The Aluminum Atom Gains Aluminum, for instance, has an atomic number of 13, which. when an atom gains an electron it gains a negative charge and is called an anion. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The mg 2 + ion, the al 3 + ion, the na +. The Aluminum Atom Gains.
From www.museoinclusivo.com
How Many Electrons Does an Aluminum Atom Have? Exploring the Atomic The Aluminum Atom Gains when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. when an atom gains an electron it gains a negative charge and is called an anion. Aluminum, for instance, has an atomic number of 13, which. in certain situations, however, the atom may lose or gain one or. The Aluminum Atom Gains.
From courses.lumenlearning.com
3.5 Periodic Variations in Element Properties General College Chemistry I The Aluminum Atom Gains to find the electron configuration of an atom, you first need to know the number of electrons that it has. the aluminum atom loses its three valence electrons. atoms of group 17 gain one electron and form anions with a 1− charge; in the particular example of aluminum, aluminum had an initial charge of zero, thanks. The Aluminum Atom Gains.
From rokanggun.blogspot.com
18+ Aluminum Atome The Aluminum Atom Gains the aluminum atom loses its three valence electrons. in the particular example of aluminum, aluminum had an initial charge of zero, thanks to the 13 electrons and 13 protons canceling one out. to find the electron configuration of an atom, you first need to know the number of electrons that it has. The mg 2 + ion,. The Aluminum Atom Gains.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table The Aluminum Atom Gains when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Atoms of group 16 gain two electrons and form ions with a 2−. when an atom gains an electron it gains a negative charge and is called an anion. in certain situations, however, the atom may lose or. The Aluminum Atom Gains.
From ar.inspiredpencil.com
Atomic Structure Of Aluminum The Aluminum Atom Gains Atoms of group 16 gain two electrons and form ions with a 2−. some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. when an atom gains an electron it gains a negative charge and is called an anion. Aluminum, for instance, has an atomic number of. The Aluminum Atom Gains.