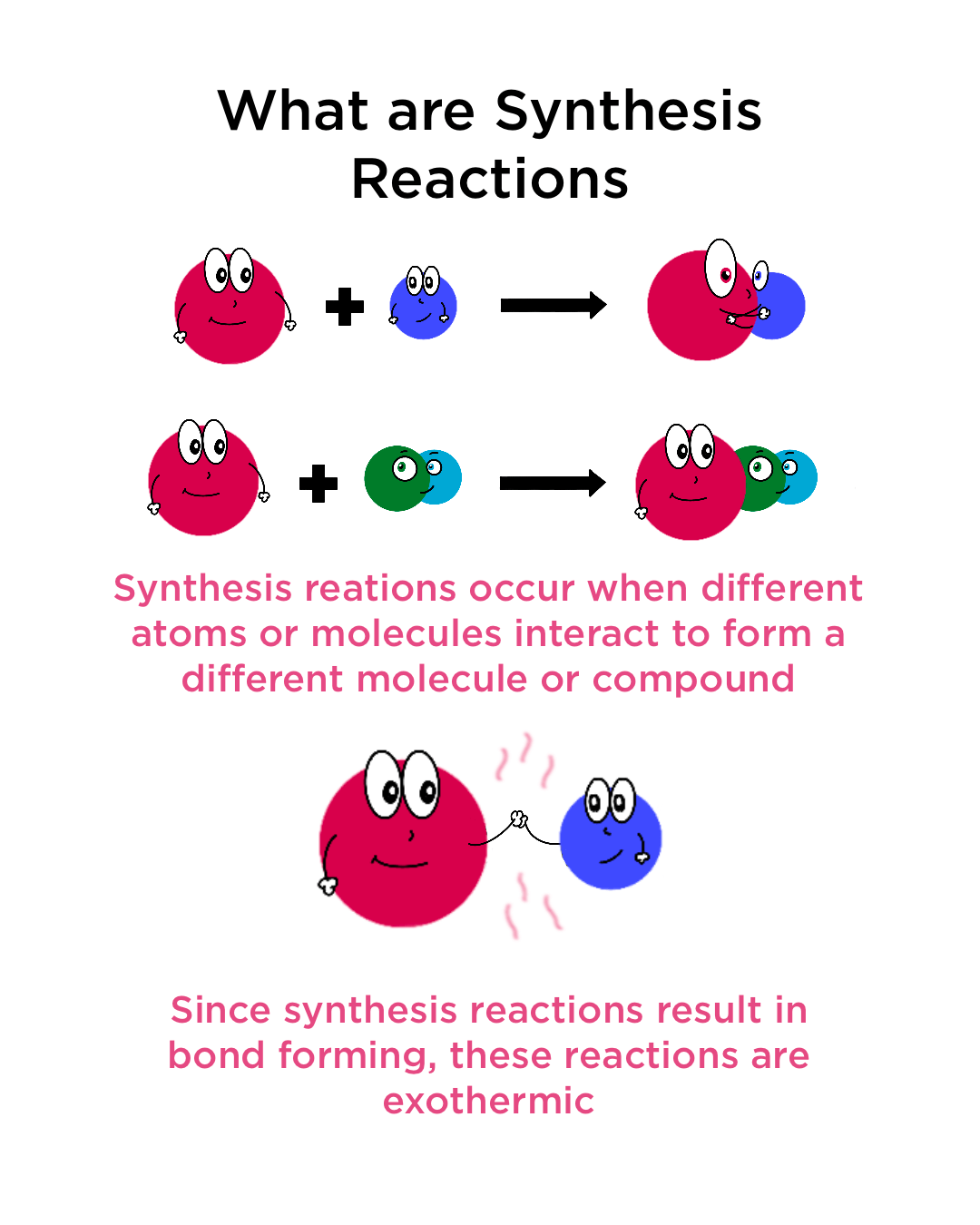
Synthesis Reaction Definition And Examples . A + b → ab. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. In a synthesis reaction, two or more chemical species combine to form a. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The two reactants combine and then form one. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. In a synthesis reaction two. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction.
from www.expii.com
A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. In a synthesis reaction, two or more chemical species combine to form a. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. In a synthesis reaction two. The two reactants combine and then form one. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. A + b → ab. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product.
Synthesis Reactions — Definition & Examples Expii
Synthesis Reaction Definition And Examples In a synthesis reaction, two or more chemical species combine to form a. In a synthesis reaction two. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. In a synthesis reaction, two or more chemical species combine to form a. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. A + b → ab. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The two reactants combine and then form one.
From www.worksheetsplanet.com
Synthesis Reaction Characteristics Synthesis Reaction Definition And Examples The two reactants combine and then form one. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A + b → ab. In a synthesis reaction, two or more. Synthesis Reaction Definition And Examples.
From www.expii.com
Synthesis Reactions — Definition & Examples Expii Synthesis Reaction Definition And Examples In a synthesis reaction, two or more chemical species combine to form a. The two reactants combine and then form one. In a synthesis reaction two. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct combination reaction is one of the. Synthesis Reaction Definition And Examples.
From www.thoughtco.com
Synthesis Reaction Definition and Examples Synthesis Reaction Definition And Examples In a synthesis reaction, two or more chemical species combine to form a. In a synthesis reaction two. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. The two reactants combine and then form one. A synthesis reaction. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT The 5 Types of Reactions PowerPoint Presentation, free download Synthesis Reaction Definition And Examples A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. Synthesis. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition And Examples A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. In a synthesis reaction, two or more chemical species combine to form a. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. Synthesis reactions are reactions that. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764 Synthesis Reaction Definition And Examples A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. In a synthesis reaction two. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Synthesis Reaction Definition And Examples Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. The two reactants combine and then form one. A synthesis reaction or direct combination reaction is a type of chemical reaction in which. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID5667577 Synthesis Reaction Definition And Examples The two reactants combine and then form one. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. In a synthesis reaction, two or more chemical species combine to form a. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. A + b. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Synthesis Reaction PowerPoint Presentation, free download ID Synthesis Reaction Definition And Examples In a synthesis reaction two. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A + b → ab. The two reactants combine and then form one. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. In a. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID Synthesis Reaction Definition And Examples A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. Synthesis reactions are reactions that involve multiple reactants reacting to form. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID6630833 Synthesis Reaction Definition And Examples Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. The two reactants combine and then form one. In a synthesis reaction two. In a synthesis reaction, two or more chemical species combine to form a. A. Synthesis Reaction Definition And Examples.
From sciencenotes.org
Types of Chemical Reactions Synthesis Reaction Definition And Examples Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A + b → ab. In a synthesis reaction two. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form. Synthesis Reaction Definition And Examples.
From www.thoughtco.com
Synthesis Reaction Description Plus Examples Synthesis Reaction Definition And Examples A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. The two reactants combine and. Synthesis Reaction Definition And Examples.
From www.youtube.com
Synthesis Reactions YouTube Synthesis Reaction Definition And Examples A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition And Examples A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. In a synthesis reaction, two or more chemical species combine to form a. Synthesis reactions are reactions that. Synthesis Reaction Definition And Examples.
From sciencenotes.org
What Is a Synthesis Reaction? Definition and Examples Synthesis Reaction Definition And Examples In a synthesis reaction, two or more chemical species combine to form a. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. In a synthesis reaction two. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more. Synthesis Reaction Definition And Examples.
From soniafersgibson.blogspot.com
Which of the Following Describes a Synthesis Reaction Synthesis Reaction Definition And Examples In a synthesis reaction two. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A + b → ab. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The two reactants combine and then. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID Synthesis Reaction Definition And Examples A + b → ab. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction.. Synthesis Reaction Definition And Examples.
From www.vrogue.co
What Is A Synthesis Reaction Definition And Examples vrogue.co Synthesis Reaction Definition And Examples Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. The two reactants combine and then form one. In a synthesis reaction two. In a synthesis reaction, two or more chemical species combine to form a. Synthesis reactions. Synthesis Reaction Definition And Examples.
From www.vrogue.co
Synthesis Reaction Definition And Examples Chemistry vrogue.co Synthesis Reaction Definition And Examples In a synthesis reaction two. A + b → ab. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. In a synthesis reaction, two or more chemical species combine to form a. Synthesis reactions (also called combination reactions). Synthesis Reaction Definition And Examples.
From ar.inspiredpencil.com
Synthesis Reaction Examples Synthesis Reaction Definition And Examples A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A + b → ab. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. A synthesis reaction or direct combination reaction is one. Synthesis Reaction Definition And Examples.
From worksheetlistfro.z13.web.core.windows.net
Synthesis Reaction Examples Chemistry Synthesis Reaction Definition And Examples A + b → ab. In a synthesis reaction two. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID Synthesis Reaction Definition And Examples A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The two reactants combine and then form one. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Synthesis reactions (also called combination reactions) are the simplest type of. Synthesis Reaction Definition And Examples.
From www.vrogue.co
Synthesis Combination Reaction Definition Examples An vrogue.co Synthesis Reaction Definition And Examples In a synthesis reaction two. A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. Synthesis reactions are reactions that involve. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Chapter 8 Chemical Equations and Reactions PowerPoint Synthesis Reaction Definition And Examples A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. In a synthesis reaction, two or more chemical species combine to form a. A + b → ab. Synthesis reactions (also called combination reactions). Synthesis Reaction Definition And Examples.
From www.dreamstime.com
Synthesis Reaction Infographic Diagram Stock Vector Illustration of Synthesis Reaction Definition And Examples Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form. Synthesis Reaction Definition And Examples.
From gbu-taganskij.ru
What Is A Synthesis Reaction? Definition And Examples, 43 OFF Synthesis Reaction Definition And Examples A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. A + b → ab. In a synthesis reaction, two or more chemical species combine to form a. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Synthesis and Reactions PowerPoint Presentation Synthesis Reaction Definition And Examples A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. In a synthesis reaction. Synthesis Reaction Definition And Examples.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Synthesis Reaction Definition And Examples In a synthesis reaction two. A + b → ab. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A synthesis reaction or. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition And Examples In a synthesis reaction two. The two reactants combine and then form one. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. In a synthesis reaction, two or more chemical species combine to form a. A +. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition And Examples A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A + b → ab. A synthesis reaction in chemistry is when two or more chemical species combine to form a more complex product. Synthesis reactions, also known as combination reactions, are a type of chemical reaction. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT CLASSIFYING CHEMICAL REACTIONS PowerPoint Presentation, free Synthesis Reaction Definition And Examples In a synthesis reaction two. The two reactants combine and then form one. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A + b → ab. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. A synthesis reaction or direct combination reaction is one of the most common types of. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Synthesis Reaction Definition And Examples The two reactants combine and then form one. Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. A + b → ab. A synthesis reaction in chemistry is when two or more. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition And Examples Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine to form a single. The two reactants combine and then form one. A + b → ab. In a synthesis reaction, two or more chemical species combine to form a. In a synthesis reaction two. A synthesis reaction or direct combination. Synthesis Reaction Definition And Examples.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Synthesis Reaction Definition And Examples The two reactants combine and then form one. A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new. In a synthesis reaction two. A + b → ab. Synthesis reactions, also known as combination reactions, are a type of chemical reaction where two or more reactants combine. Synthesis Reaction Definition And Examples.