Aluminum Bromide And Chlorine Balanced Equation . Let's balance this equation using the inspection method. First, we set all coefficients to 1: 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. In order to sum to zero, you need to have three. Explain the roles of subscripts and coefficients in chemical equations. What is the balanced chemical equation for this reaction: Balance a chemical equation when given the unbalanced equation. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid.
from us.metoree.com
The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. What is the balanced chemical equation for this reaction: Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. First, we set all coefficients to 1: Explain the roles of subscripts and coefficients in chemical equations. In order to sum to zero, you need to have three. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,.
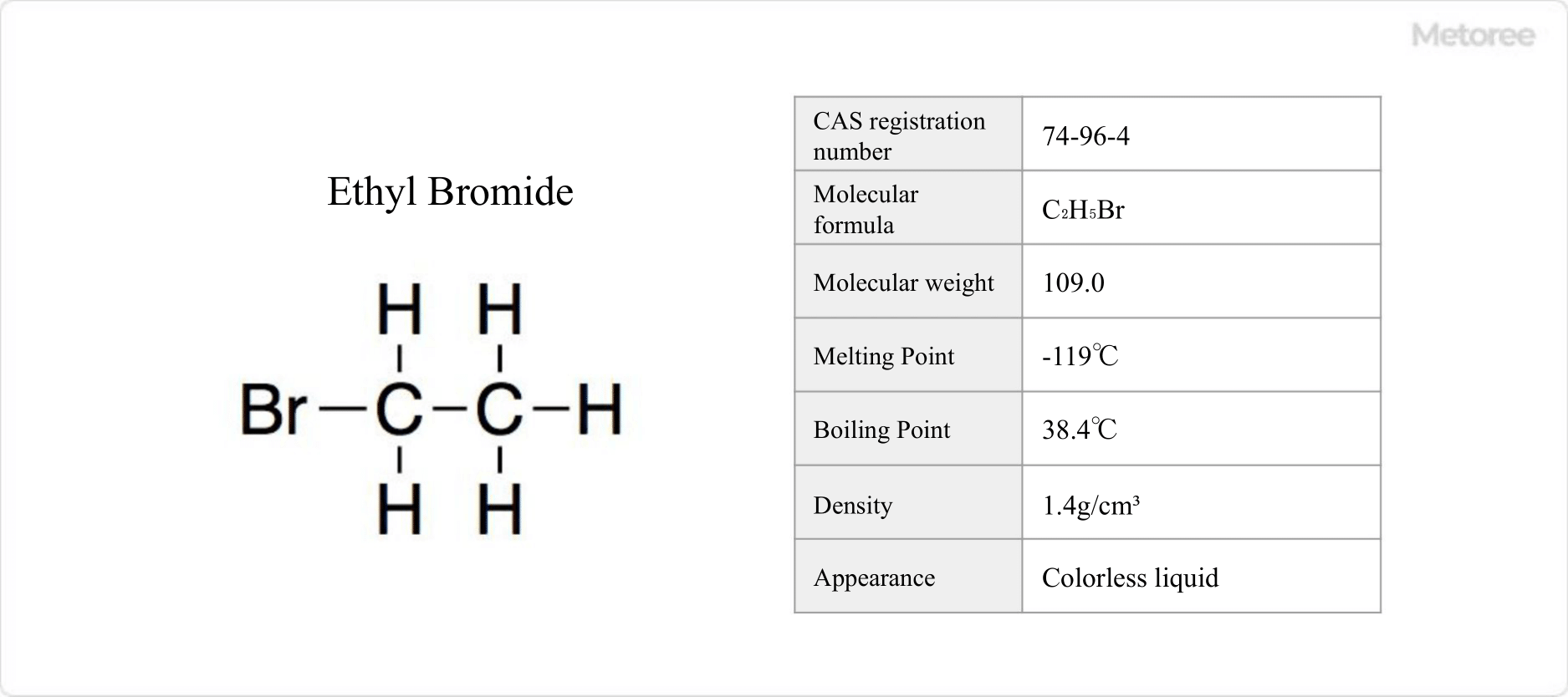
41 Ethyl Bromide Manufacturers in 2024 Metoree
Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Balance a chemical equation when given the unbalanced equation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. First, we set all coefficients to 1: 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. In order to sum to zero, you need to have three. Explain the roles of subscripts and coefficients in chemical equations. What is the balanced chemical equation for this reaction: Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Let's balance this equation using the inspection method.
From www.numerade.com
SOLVED 1. Balance the following equations without using the model set AlBr, KSO, AL(SO ), Se 0 Aluminum Bromide And Chlorine Balanced Equation In order to sum to zero, you need to have three. First, we set all coefficients to 1: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. What is the balanced chemical equation for this reaction: Explain the roles of subscripts and coefficients in chemical equations.. Aluminum Bromide And Chlorine Balanced Equation.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Aluminum Bromide And Chlorine Balanced Equation What is the balanced chemical equation for this reaction: Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where. Aluminum Bromide And Chlorine Balanced Equation.
From slideplayer.com
Chapter 8 Chemical Reactions ppt download Aluminum Bromide And Chlorine Balanced Equation Let's balance this equation using the inspection method. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. The chemical equation described in section 4.1 is balanced, meaning. Aluminum Bromide And Chlorine Balanced Equation.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine. YouTube Aluminum Bromide And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Gaseous chlorine reacts with an aqueous solution of potassium. Aluminum Bromide And Chlorine Balanced Equation.
From slideplayer.com
Chemical Reactions. ppt download Aluminum Bromide And Chlorine Balanced Equation Let's balance this equation using the inspection method. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Explain the roles of subscripts and coefficients in chemical equations. What is the balanced chemical equation for this reaction: First, we set all coefficients to 1: Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction. Aluminum Bromide And Chlorine Balanced Equation.
From www.youtube.com
How to Balance Al + Br2 = AlBr3 (Aluminum + Bromine gas) YouTube Aluminum Bromide And Chlorine Balanced Equation In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. What is the balanced chemical equation for this reaction: Let's balance this equation using the inspection method. 1 albr 3 + 1 cl 2 =. Aluminum Bromide And Chlorine Balanced Equation.
From slideplayer.com
Types of Chemical Reactions ppt download Aluminum Bromide And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Let's balance this equation using the inspection method. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Balance a chemical equation when given the unbalanced equation. What is the balanced chemical equation for this reaction:. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Word Equations Write the word equations below as chemical equations and balance; zinc Aluminum Bromide And Chlorine Balanced Equation In order to sum to zero, you need to have three. Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. The. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVEDThe balanced equation for the reaction of aluminum metal and chlorine gas is 2 Al(s)+3 Aluminum Bromide And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. First, we set all coefficients to 1: Let's balance this equation using the inspection method. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Gaseous chlorine reacts with an aqueous solution of. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Questions Balance the following equations without using the model set AlBr, KSO4, SO2 Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Explain the roles of subscripts and coefficients in chemical equations. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Balance a chemical equation when given the unbalanced equation. Albr3 + cl = alcl3 + br2 is a single. Aluminum Bromide And Chlorine Balanced Equation.
From www.chegg.com
Solved Write the word equations below, predict the product, Aluminum Bromide And Chlorine Balanced Equation Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. In order to sum to zero, you need to have three. Explain the. Aluminum Bromide And Chlorine Balanced Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and chlorine gas react to form Aluminum Bromide And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. First, we set all coefficients to 1: Let's balance this. Aluminum Bromide And Chlorine Balanced Equation.
From www.youtube.com
Ethene + Hydrogen Bromide (Reaction and Mechanism) YouTube Aluminum Bromide And Chlorine Balanced Equation Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. First, we set all coefficients to 1: Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are.. Aluminum Bromide And Chlorine Balanced Equation.
From studyx.ai
Name qquad Writing Balanced Chemical StudyX Aluminum Bromide And Chlorine Balanced Equation First, we set all coefficients to 1: Let's balance this equation using the inspection method. Balance a chemical equation when given the unbalanced equation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Explain the roles of subscripts and coefficients in chemical equations. In order to. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Balance a chemical equation when. Aluminum Bromide And Chlorine Balanced Equation.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Aluminum Bromide And Chlorine Balanced Equation What is the balanced chemical equation for this reaction: In order to sum to zero, you need to have three. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3]. Aluminum Bromide And Chlorine Balanced Equation.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Illustration of stuff, alcohol Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Albr3 + cl = alcl3. Aluminum Bromide And Chlorine Balanced Equation.
From byjus.com
Mechanism of Hoffman bromide degradation reaction Aluminum Bromide And Chlorine Balanced Equation In order to sum to zero, you need to have three. What is the balanced chemical equation for this reaction: 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Aluminum Bromide And Chlorine Balanced Equation First, we set all coefficients to 1: Explain the roles of subscripts and coefficients in chemical equations. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. What is the balanced chemical equation for this reaction: Let's balance this equation using the inspection method. The chemical. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED A. Write balanced chemical equations Mercury (II) chloride + aluminum acetate Zinc Aluminum Bromide And Chlorine Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. In order to sum to zero, you need to have three. Let's balance this equation using the inspection method. Balance a chemical equation when given the unbalanced equation. Albr3 + cl = alcl3 + br2 is a. Aluminum Bromide And Chlorine Balanced Equation.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Illustration Illustration of Aluminum Bromide And Chlorine Balanced Equation Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. First, we set all coefficients to 1: Balance a chemical equation when given the unbalanced equation. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. In order to sum to zero, you. Aluminum Bromide And Chlorine Balanced Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chloride+Bromine YouTube Aluminum Bromide And Chlorine Balanced Equation Let's balance this equation using the inspection method. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Albr3 +. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVEDPart III Mass Relationships in Chemical Reactions (Stoichiometry) Aluminum metal reacts Aluminum Bromide And Chlorine Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Explain the roles of subscripts and coefficients in chemical equations. Let's balance this equation using the inspection method. Balance a chemical equation when given the unbalanced equation. 1 albr 3 + 1 cl 2 = 1 alcl. Aluminum Bromide And Chlorine Balanced Equation.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Aluminum Bromide And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Let's balance this equation using the inspection method. What is the balanced chemical equation for this reaction: Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Gaseous chlorine reacts with an aqueous solution of potassium. Aluminum Bromide And Chlorine Balanced Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free download ID2276630 Aluminum Bromide And Chlorine Balanced Equation What is the balanced chemical equation for this reaction: Balance a chemical equation when given the unbalanced equation. Let's balance this equation using the inspection method. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. In order to sum to zero, you need to have. Aluminum Bromide And Chlorine Balanced Equation.
From slideplayer.com
All About Equations Predict This Keep Your Balance Identify Me ppt download Aluminum Bromide And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. What is the balanced chemical equation for this reaction: Let's balance this equation using the inspection method. Gaseous chlorine reacts with an aqueous solution of potassium. Aluminum Bromide And Chlorine Balanced Equation.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. In order to sum to zero, you need to have three. 1 albr 3 + 1 cl 2 = 1 alcl 3 +. Aluminum Bromide And Chlorine Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free download ID4396540 Aluminum Bromide And Chlorine Balanced Equation First, we set all coefficients to 1: Let's balance this equation using the inspection method. In order to sum to zero, you need to have three. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section. Aluminum Bromide And Chlorine Balanced Equation.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Aluminum Bromide And Chlorine Balanced Equation Let's balance this equation using the inspection method. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that. Aluminum Bromide And Chlorine Balanced Equation.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Aluminum Bromide And Chlorine Balanced Equation Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. Explain the roles of subscripts and coefficients in chemical equations. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. The chemical equation described in section 4.1 is. Aluminum Bromide And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Write and balance a chemical equation describing the following reactions Aluminum metal Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. What is the balanced chemical equation for this reaction: Balance a chemical equation when. Aluminum Bromide And Chlorine Balanced Equation.
From askfilo.com
Problem 3 Aluminum reacts with chlorine gas to form aluminum chloride v.. Aluminum Bromide And Chlorine Balanced Equation What is the balanced chemical equation for this reaction: Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Balance a chemical equation when given the unbalanced equation. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. The chemical equation described in section 4.1 is balanced, meaning that. Aluminum Bromide And Chlorine Balanced Equation.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Aluminum Bromide And Chlorine Balanced Equation Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. Let's balance this equation using the inspection method. 1 albr 3 + 1 cl 2 = 1 alcl 3 + 1 br 2 for each element,. First, we set all coefficients to 1: Balance a chemical equation when given the unbalanced equation. In order to sum to. Aluminum Bromide And Chlorine Balanced Equation.
From sielc.com
Vinyl bromide SIELC Aluminum Bromide And Chlorine Balanced Equation In order to sum to zero, you need to have three. Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Balance a chemical equation when given the unbalanced equation. What is the balanced. Aluminum Bromide And Chlorine Balanced Equation.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector (Royalty Free) 1977753731 Aluminum Bromide And Chlorine Balanced Equation Balance a chemical equation when given the unbalanced equation. In order to sum to zero, you need to have three. Albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide [albr 3] and six. What is the balanced chemical equation for this reaction: First, we set all coefficients to 1:. Aluminum Bromide And Chlorine Balanced Equation.