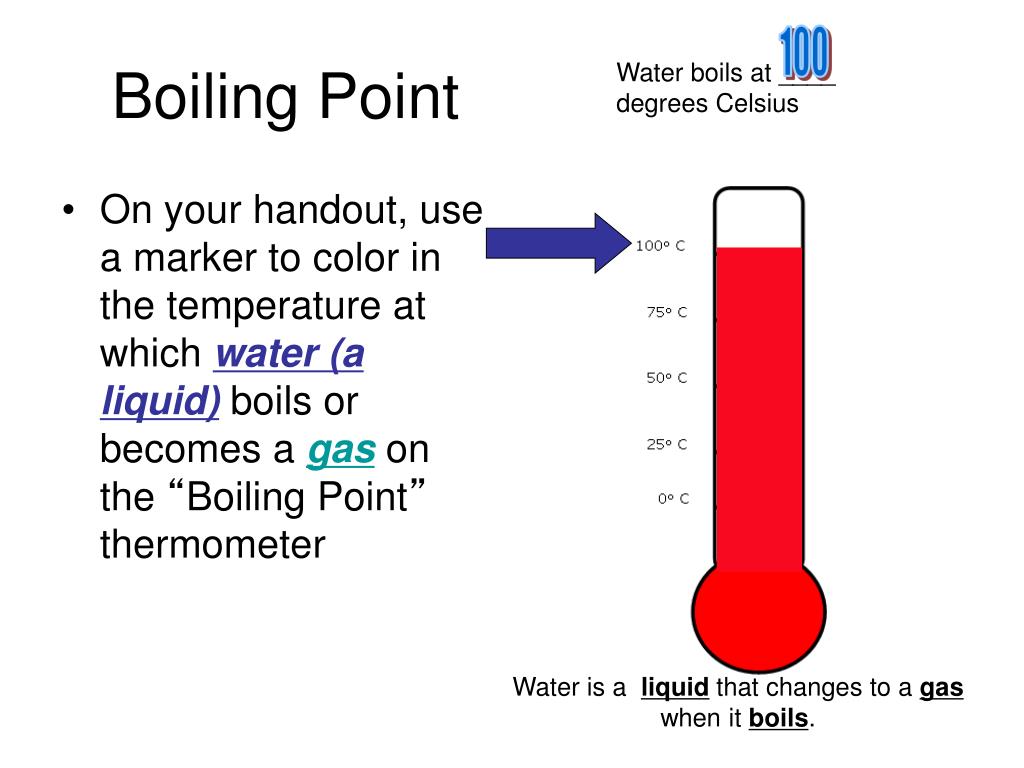
What Is The Effect Of Adding Sucrose To The Boiling Point Of Water . Adding 1 gram of sugar, or any other substance that does not create ions, increases. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. Adding sugar to water increases the boiling point. According to table 13.8.1 13.8. It is easily possible that some of this dimer disassociates. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. 1, the molal boiling point elevation constant for water is 0.51°c/m. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Note that the normal boiling. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute.
from www.slideserve.com
It is easily possible that some of this dimer disassociates. Note that the normal boiling. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Adding 1 gram of sugar, or any other substance that does not create ions, increases. According to table 13.8.1 13.8. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding sugar to water increases the boiling point. 1, the molal boiling point elevation constant for water is 0.51°c/m.
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free download ID2755993
What Is The Effect Of Adding Sucrose To The Boiling Point Of Water A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. According to table 13.8.1 13.8. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Note that the normal boiling. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Adding sugar to water increases the boiling point. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. 1, the molal boiling point elevation constant for water is 0.51°c/m. It is easily possible that some of this dimer disassociates. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6009487 What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. It is easily possible that some of this dimer disassociates. According to table 13.8.1 13.8. Note that the normal boiling. 1, the molal boiling point elevation constant for. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.meritnation.com
The boiling point of water at 750 mm Hg is 96 63 C How much sucrose is to be Chemistry What Is The Effect Of Adding Sucrose To The Boiling Point Of Water According to table 13.8.1 13.8. It is easily possible that some of this dimer disassociates. 1, the molal boiling point elevation constant for water is 0.51°c/m. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. It is easily possible that some of this dimer disassociates. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. According to table 13.8.1 13.8. Adding sugar. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Note that the normal boiling. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. A 1 m aqueous solution of sucrose (342 g/mol) and a. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED What is the boiling point of a solution made using 819 g of sucrose, C12H22O11, in 0.275 What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Adding sugar to water increases the boiling point. Note that the normal boiling. It is easily possible that some of this dimer disassociates. 1, the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From mavink.com
Melting And Boiling Point Chart What Is The Effect Of Adding Sucrose To The Boiling Point Of Water According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. Almost 2000 g will dissolve in 1. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED Experiment 1 Effect of Solutes on the Boiling and Freezing Points of Water PreLab What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. A 1 m aqueous solution of sucrose (342 g/mol). What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From vanessa-loksellers.blogspot.com
VanessaLokSellers What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the boiling point. 1, the molal boiling point elevation constant for water is 0.51°c/m. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.youtube.com
Equation for C12H22O11 + H2O (Sucrose + Water) YouTube What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Note that the normal boiling. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. According to table 13.8.1 13.8. It is easily possible that some of this dimer disassociates. Adding 1 gram of sugar, or any other substance that does not create ions, increases. A 1 m aqueous solution of. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED Part A. What is the boiling point of a solution made using 733 g of sucrose, C12H22O11 What Is The Effect Of Adding Sucrose To The Boiling Point Of Water 1, the molal boiling point elevation constant for water is 0.51°c/m. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding sugar to water increases the boiling point. Note that the normal boiling. According to table. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From studylib.net
Boiling point elevation of sucrose solutions What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. 1, the molal boiling point elevation constant for water is 0.51°c/m. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED Cowboy coffee" is prepared by adding coffee grounds to boiling water. If 12.5 g sucrose What Is The Effect Of Adding Sucrose To The Boiling Point Of Water A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. 1, the molal boiling point elevation constant for water is 0.51°c/m. Almost 2000 g will dissolve in 1 l of water, giving rise. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID4365473 What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Note that the normal boiling. It is easily possible that some of. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.researchgate.net
Sucrose composition as a function of boiling point Download Table What Is The Effect Of Adding Sucrose To The Boiling Point Of Water According to table 13.8.1 13.8. Adding 1 gram of sugar, or any other substance that does not create ions, increases. It is easily possible that some of this dimer disassociates. 1, the molal boiling point elevation constant for water is 0.51°c/m. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. Adding sugar to water lowers the. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED Two moles of sucrose are dissolved in 8 moles of water. Assume that the solution follows What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Note that the normal boiling. Adding sugar to water lowers the freezing point because the sugar molecules. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.researchgate.net
SucroseWater Phase Equilibrium Diagram (Mathlouthi and Reiser, 1995)... Download Scientific What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Note that the normal boiling. It is easily possible that some of this dimer disassociates. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. According to table 13.8.1 13.8. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m.. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From brainly.com
How do solve for 13?What is the boiling point of a solution made by dissolving 1.0000 mole of What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Adding 1 gram of sugar, or any other substance that does not create ions, increases. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. Almost 2000 g will dissolve in 1 l. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free download ID2755993 What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Note that the normal boiling. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. Thus a 1.00 m aqueous solution of a nonvolatile molecular. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED Which solution will have a higher boiling point A solution containing 105 grams of What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water increases the boiling point. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Adding 1 gram of sugar, or any other substance. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED What is the boiling point of a solution of 115.0 g of sucrose, C12H22O11, in 350.0 g of What Is The Effect Of Adding Sucrose To The Boiling Point Of Water 1, the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. Adding sugar to water increases the boiling point. Thus a 1.00 m aqueous solution of a nonvolatile. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is The Effect Of Adding Sucrose To The Boiling Point Of Water It is easily possible that some of this dimer disassociates. Note that the normal boiling. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. A 1 m aqueous solution of sucrose (342 g/mol) and a 1. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Effect Of Adding Sucrose To The Boiling Point Of Water 1, the molal boiling point elevation constant for water is 0.51°c/m. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. Note that the normal boiling. According to table 13.8.1 13.8. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. According to table \(\pageindex{1}\), the molal boiling point. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From askfilo.com
12 What is the boiling point of 0.5 molal aqueous solution of sucrose if What Is The Effect Of Adding Sucrose To The Boiling Point Of Water It is easily possible that some of this dimer disassociates. Note that the normal boiling. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Adding 1 gram of sugar, or any other substance that does not create ions, increases. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
If 0.0125 mol of sucrose, C12H22O11, are dissolved in 15.2 grams of water, what will be the What Is The Effect Of Adding Sucrose To The Boiling Point Of Water 1, the molal boiling point elevation constant for water is 0.51°c/m. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. According to table 13.8.1 13.8. Adding 1. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Effect Of Adding Sucrose To The Boiling Point Of Water According to table 13.8.1 13.8. Adding sugar to water increases the boiling point. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. 1, the. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.youtube.com
If for the sucrose solution elevation in boiling point is `0.1^ YouTube What Is The Effect Of Adding Sucrose To The Boiling Point Of Water A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water increases the boiling point. Adding sugar to water lowers the freezing point because the sugar molecules. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From foodcrumbles.com
Phase Diagrams Explained Demystifying Ice Cream FoodCrumbles What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. 1, the molal boiling point elevation constant for water is 0.51°c/m. According to table 13.8.1 13.8. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.toppr.com
The difference between the boiling point and freezing point of an aqueous solution containing What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. It is easily possible that some of this dimer disassociates. According to table 13.8.1 13.8. 1, the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water increases the boiling point. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID4365473 What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such as. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water lowers the. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From byjus.com
boiling point of water at 750 mm Hg is 99.63 ^oC .How much sugar is to be added to 500 g of What Is The Effect Of Adding Sucrose To The Boiling Point Of Water 1, the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. A. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.youtube.com
Boiling point of water at 750mm Hg is 99.63oC. How much sucrose is to be added to 500g of water What Is The Effect Of Adding Sucrose To The Boiling Point Of Water According to table 13.8.1 13.8. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. It is easily possible that some of this dimer disassociates. 1, the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water increases the boiling point. Adding. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From geovanniferskoch.blogspot.com
How Can You Increase the Solubility of Sugar in Water What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the boiling point. A 1 m aqueous solution of sucrose (342 g/mol) and a 1 m aqueous solution of ethylene glycol (62 g/mol) will exhibit the same boiling point. According to table \(\pageindex{1}\), the molal boiling point elevation constant for. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.youtube.com
Ncert Solution intext 2.10 Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Thus a 1.00 m aqueous solution of a nonvolatile molecular solute. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Note that the normal boiling. Almost 2000 g will dissolve in 1 l of water, giving rise to what amounts to pancake syrup. Adding sugar to water increases the boiling. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED For a 0.50 molal solution of sucrose in water, calculate the freezing point and the What Is The Effect Of Adding Sucrose To The Boiling Point Of Water Adding sugar to water increases the boiling point. According to table 13.8.1 13.8. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Note that the normal boiling. It is easily possible that some of this dimer disassociates. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.
From www.numerade.com
SOLVED An aqueous sucrose solution (342.30 g/mol) has a boiling point increase of 0.39 °C What Is The Effect Of Adding Sucrose To The Boiling Point Of Water According to table \(\pageindex{1}\), the molal boiling point elevation constant for water is 0.51°c/m. 1, the molal boiling point elevation constant for water is 0.51°c/m. Adding sugar to water increases the boiling point. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Thus a 1.00 m aqueous solution of a nonvolatile molecular solute such. What Is The Effect Of Adding Sucrose To The Boiling Point Of Water.