Beer's Law Post Lab Questions . In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Solution preparation and beer’s law post lab report questions i. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculations (show how you calculated the bulleted items; Include units and the correct number of significant figures.). Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? This relationship is called the. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. The method involving the volumetric flask is the most accurate and precise.
from www.chegg.com
Include units and the correct number of significant figures.). The method involving the volumetric flask is the most accurate and precise. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculations (show how you calculated the bulleted items; The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Solution preparation and beer’s law post lab report questions i. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets?
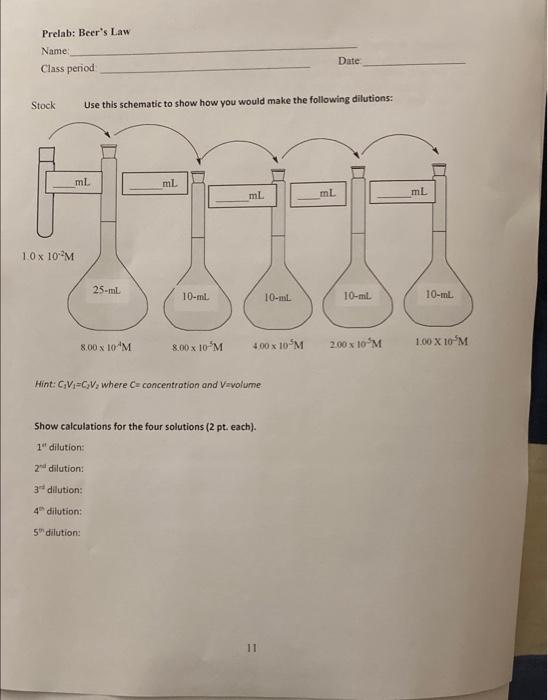
Solved Prelab Beer's Law Name Class period Date Stock Use
Beer's Law Post Lab Questions Include units and the correct number of significant figures.). Calculations (show how you calculated the bulleted items; This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Include units and the correct number of significant figures.). Solution preparation and beer’s law post lab report questions i. The method involving the volumetric flask is the most accurate and precise. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Post Lab Questions Solution preparation and beer’s law post lab report questions i. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculations (show how you calculated the bulleted items; This relationship is called. Beer's Law Post Lab Questions.
From webgiasi.vn
Beer’s Law Calculating Concentration from Absorbance กฎ ของ beer Beer's Law Post Lab Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The method involving the volumetric flask is the most accurate and precise. This relationship is called the. Include units and the correct number of significant figures.). Why is it necessary to rinse the pipets with the solution to be used in them before using. Beer's Law Post Lab Questions.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law Beer's Law Post Lab Questions Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? Calculations (show how you calculated the bulleted items; The method involving the volumetric flask is the most accurate and precise. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Report sheets Exp. 4 Beer's Law Suggested DATA TABLE Beer's Law Post Lab Questions Calculations (show how you calculated the bulleted items; Solution preparation and beer’s law post lab report questions i. The method involving the volumetric flask is the most accurate and precise. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Why is it necessary to rinse the pipets. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Lab 5 2162 Beer's Law and Standard Curves Prepared by Beer's Law Post Lab Questions Include units and the correct number of significant figures.). Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. The amount of. Beer's Law Post Lab Questions.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer's Law Post Lab Questions Include units and the correct number of significant figures.). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? This relationship is called the. Solution preparation and beer’s. Beer's Law Post Lab Questions.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Beer's Law Post Lab Questions The method involving the volumetric flask is the most accurate and precise. Calculations (show how you calculated the bulleted items; Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. This relationship is called the. Why is it necessary to rinse the. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Experiment 12 Beer's Law PreLab Report Sheet Name Beer's Law Post Lab Questions Solution preparation and beer’s law post lab report questions i. Calculations (show how you calculated the bulleted items; Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Name Experiment 6 Beer's Law Pre Lab Questions 1. Beer's Law Post Lab Questions Include units and the correct number of significant figures.). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculations (show how you calculated the bulleted items; This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.. Beer's Law Post Lab Questions.
From oneclass.com
OneClass Determining the Concentration of a Solution Beer's Law Beer's Law Post Lab Questions This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The method involving the volumetric flask is the most accurate and precise. Calculations (show how you calculated the bulleted items; Why is it necessary to rinse the pipets with the solution to be. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Experiment V Equilibrium Constant PostLab Questions Beer's Law Post Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Solution preparation and beer’s law post lab report questions i. Calculations (show how you calculated the bulleted items; In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called. Beer's Law Post Lab Questions.
From www.chegg.com
Solved PHY 131 Lab 9 field in a solenoid Using Beer's Law Post Lab Questions This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Include units and the correct number of significant figures.). The method involving the volumetric flask is the most accurate and precise. Calculations (show how you calculated the bulleted items; The amount of light that a species absorbs in a. Beer's Law Post Lab Questions.
From www.chegg.com
and Hess's Law PostLab Questions Lab 9 1. In the Beer's Law Post Lab Questions The method involving the volumetric flask is the most accurate and precise. Include units and the correct number of significant figures.). Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. Calculations (show how you calculated the bulleted items; Why is it. Beer's Law Post Lab Questions.
From www.thinkswap.com
Lab Report Beer's law and standard curve for coloured compounds BMC Beer's Law Post Lab Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Include units and the correct number of significant figures.). The method involving the volumetric flask is the most accurate and precise. This relationship is called the. Why is it necessary to rinse the pipets with the solution to be used in them before using. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Prelab Beer's Law Name Class period Date Stock Use Beer's Law Post Lab Questions Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b). Beer's Law Post Lab Questions.
From studylib.net
Beer's Law Lab Beer's Law Post Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Include units and the correct number of significant figures.). Calculations (show how you calculated the bulleted items; Solution preparation and beer’s law post lab report questions i. Beer’s law refers to the amount. Beer's Law Post Lab Questions.
From www.chegg.com
Solved PreLab Assignment Beer's Law This experiment covers Beer's Law Post Lab Questions Include units and the correct number of significant figures.). This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculations (show how you calculated the bulleted items; The method involving the volumetric flask is the most accurate and precise. Beer’s law refers to. Beer's Law Post Lab Questions.
From studylib.net
Beer's_Law_Lab_with_Analysis Beer's Law Post Lab Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Solution preparation. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Post Lab Questions Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The method involving the volumetric flask is the most accurate and precise. Solution preparation and beer’s. Beer's Law Post Lab Questions.
From www.softpedia.com
Download Beer's Law Lab Beer's Law Post Lab Questions Include units and the correct number of significant figures.). The method involving the volumetric flask is the most accurate and precise. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. Why is it necessary to rinse the pipets with the solution to be used in them before using. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Post Lab Questions Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? Solution preparation and beer’s law post lab report questions i. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The method involving. Beer's Law Post Lab Questions.
From studylib.net
Beer's Law Lab Beer's Law Post Lab Questions This relationship is called the. Calculations (show how you calculated the bulleted items; Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Solution preparation and. Beer's Law Post Lab Questions.
From studylib.net
Lab 7 Beer's Law Beer's Law Post Lab Questions Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? Calculations (show how you calculated the bulleted items; The amount of light. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Post Lab Questions This relationship is called the. The method involving the volumetric flask is the most accurate and precise. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? Solution preparation and beer’s law post lab report questions i. The amount of light that a species absorbs in a spectroscopic transition can. Beer's Law Post Lab Questions.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Post Lab Questions Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? The method involving the volumetric flask is the most accurate and precise. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute.. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Post Lab Questions The method involving the volumetric flask is the most accurate and precise. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Why is it necessary to rinse the pipets with the. Beer's Law Post Lab Questions.
From www.studypool.com
SOLUTION CHEM 025 Beers Law Virtual Lab Report Studypool Beer's Law Post Lab Questions Include units and the correct number of significant figures.). This relationship is called the. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The method. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Post Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The method involving the volumetric flask is the most accurate and precise. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law refers to the amount of energy absorbed by. Beer's Law Post Lab Questions.
From www.chegg.com
Solved Prelab LO7 Beer's Law (10pts) Name Please review the Beer's Law Post Lab Questions Include units and the correct number of significant figures.). Solution preparation and beer’s law post lab report questions i. Calculations (show how you calculated the bulleted items; Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. The method involving the volumetric. Beer's Law Post Lab Questions.
From studylib.net
Beer's Law Lab Beer's Law Post Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The method involving the volumetric flask is the most accurate and precise. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. Include units and the correct. Beer's Law Post Lab Questions.
From www.youtube.com
Beer’s Law Lab Analysis YouTube Beer's Law Post Lab Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions. Beer's Law Post Lab Questions.
From www.chegg.com
Solved POSTLAB QUESTIONS Spectroscopy and Concentration Beer's Law Post Lab Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? The method involving the volumetric flask is the most accurate and precise. Beer’s law refers to the amount of energy absorbed by a solution. Beer's Law Post Lab Questions.
From www.chegg.com
Solved PRE?LAB QUESTIONS 1. Write the chemical reaction Beer's Law Post Lab Questions The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Include units and the correct number of significant figures.). Calculations (show how you calculated the bulleted items;. Beer's Law Post Lab Questions.
From www.studocu.com
Beer’s Law PreLab Worksheet Complete the worksheet and submit it to Beer's Law Post Lab Questions The method involving the volumetric flask is the most accurate and precise. Why is it necessary to rinse the pipets with the solution to be used in them before using the pipets? Solution preparation and beer’s law post lab report questions i. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Calculations (show. Beer's Law Post Lab Questions.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Post Lab Questions This relationship is called the. Beer’s law refers to the amount of energy absorbed by a solution being proportional to the solutions molar absorbency as well as the concentration of the current solute. The method involving the volumetric flask is the most accurate and precise. Calculations (show how you calculated the bulleted items; Solution preparation and beer’s law post lab. Beer's Law Post Lab Questions.