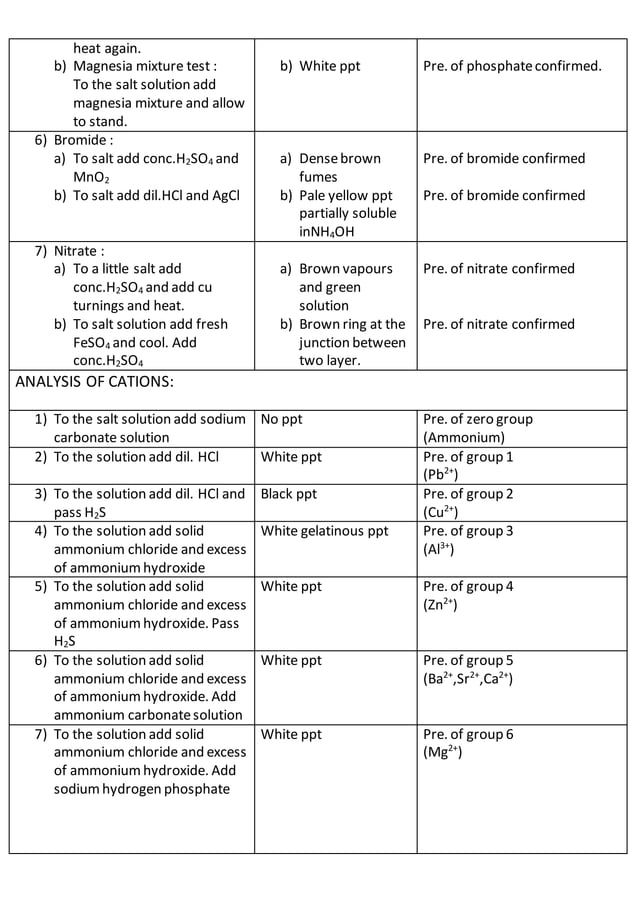
Common Salts Given For Salt Analysis . Boil, then add solid ki + fresh starch sol = deep blue. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. This experiment involves identifying the cations and anions in various salt solutions. Chemists often have to identify the composition of unknown substances. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. We + dil h2so4 (or dil acetic acid);
from www.slideshare.net
We + dil h2so4 (or dil acetic acid); Chemists often have to identify the composition of unknown substances. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Boil, then add solid ki + fresh starch sol = deep blue. This experiment involves identifying the cations and anions in various salt solutions. The common ion effect is most. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis.
CHEMISTRY Salt analysis class 12
Common Salts Given For Salt Analysis The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. This experiment involves identifying the cations and anions in various salt solutions. Boil, then add solid ki + fresh starch sol = deep blue. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. The common ion effect is most. Chemists often have to identify the composition of unknown substances. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. We + dil h2so4 (or dil acetic acid);
From www.slideshare.net
Colours of some common salts compounds Common Salts Given For Salt Analysis Boil, then add solid ki + fresh starch sol = deep blue. The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. We + dil h2so4 (or dil acetic acid); The segregation of different anions and cations and identification of the same in inorganic salts is. Common Salts Given For Salt Analysis.
From sciphychem.blogspot.com
SciPhyChem (O level Chem) How to prepare salts Common Salts Given For Salt Analysis This experiment involves identifying the cations and anions in various salt solutions. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Boil, then add solid ki + fresh starch sol = deep blue. Qualitative salt analysis is the process of determining the number of constituents present in an. Common Salts Given For Salt Analysis.
From www.sliderbase.com
Precipitation Reactions Presentation Chemistry Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. We + dil h2so4 (or dil acetic acid); This experiment involves identifying the cations and anions in various salt solutions. The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt. Common Salts Given For Salt Analysis.
From www.scribd.com
Chemistry Salt Analysis Cheatsheet Chemical Substances Chemical Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions and cations and identification of the. Common Salts Given For Salt Analysis.
From www.youtube.com
Salt Analysis How to Perform How to Write in Practical Journal Common Salts Given For Salt Analysis Boil, then add solid ki + fresh starch sol = deep blue. We + dil h2so4 (or dil acetic acid); The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. Chemists often have to identify the composition of unknown substances. The common ion effect is most. Qualitative analysis of inorganic. Common Salts Given For Salt Analysis.
From www.youtube.com
Importance of common saltUses of common saltEssay on common salt Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt. Common Salts Given For Salt Analysis.
From www.slideshare.net
CHEMISTRY Salt analysis class 12 Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. This experiment involves identifying the cations and anions in various salt solutions. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a. Common Salts Given For Salt Analysis.
From www.labkafe.com
Color of Common Salts Used in School Laboratories Labkafe Common Salts Given For Salt Analysis This experiment involves identifying the cations and anions in various salt solutions. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. We + dil h2so4 (or dil acetic acid); Chemists often have to identify the composition of unknown substances. Boil, then add solid ki + fresh starch sol =. Common Salts Given For Salt Analysis.
From www.scribd.com
Salt Analysis Procedure PDF Common Salts Given For Salt Analysis The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. The common ion effect is most. This experiment involves identifying the cations and anions in various salt solutions. Chemists often have to identify the composition of unknown substances. Qualitative analysis of inorganic salts means the identification of cations and anions. Common Salts Given For Salt Analysis.
From learnchemistrysaltform4.weebly.com
Qualitative analysis of salts Learn Chemistry Corner Common Salts Given For Salt Analysis Boil, then add solid ki + fresh starch sol = deep blue. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. We + dil h2so4 (or dil acetic. Common Salts Given For Salt Analysis.
From chemistrycalc.com
Salt Analysis Formulas Complete List Formulae Sheet for Salt Analysis Common Salts Given For Salt Analysis The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. We + dil h2so4 (or dil acetic acid); Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Qualitative salt analysis is the process of determining the number of. Common Salts Given For Salt Analysis.
From chemistrycalc.com
Salt Analysis Formulas Complete List Formulae Sheet for Salt Analysis Common Salts Given For Salt Analysis The common ion effect is most. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. Boil, then add solid ki + fresh starch sol = deep blue. We + dil h2so4 (or dil acetic acid); Qualitative analysis of inorganic salts means the identification of cations and anions present in. Common Salts Given For Salt Analysis.
From www.youtube.com
Salt Analysis YouTube Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Chemists often have to identify the composition of unknown substances. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. The common ion effect is most. This experiment involves identifying the. Common Salts Given For Salt Analysis.
From www.pinterest.com
Salt analysis Teaching chemistry, Chemistry basics, Chemistry lessons Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. We + dil h2so4 (or dil acetic acid); Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Boil, then add. Common Salts Given For Salt Analysis.
From www.slideshare.net
CHEMISTRY Salt analysis class 12 Common Salts Given For Salt Analysis This experiment involves identifying the cations and anions in various salt solutions. The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. We + dil h2so4. Common Salts Given For Salt Analysis.
From www.youtube.com
Salt Definition, Formation, Examples, Properties and Uses Some Common Common Salts Given For Salt Analysis Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. This experiment involves identifying the cations and anions in various salt solutions. The common ion effect is most. We + dil. Common Salts Given For Salt Analysis.
From www.slideshare.net
Chapter 8 SALTS Common Salts Given For Salt Analysis The common ion effect is most. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. Chemists often have to identify the composition of unknown substances. We + dil h2so4 (or dil acetic acid); This experiment involves identifying the cations and anions in various salt solutions. Qualitative analysis of inorganic. Common Salts Given For Salt Analysis.
From www.scribd.com
Salt Analysis Chart Materials Chemical Substances Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Boil, then add solid ki + fresh starch sol = deep blue. Chemists often have to identify the composition of unknown substances. This experiment involves identifying the cations and anions in various salt solutions. The segregation of different anions and cations and. Common Salts Given For Salt Analysis.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. This experiment involves identifying the cations and anions in various salt solutions. The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Qualitative analysis of inorganic salts means the identification of cations and anions present in. Common Salts Given For Salt Analysis.
From byjus.com
How to memorize the colour of all the salts???? Common Salts Given For Salt Analysis Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Chemists often have to identify the composition of unknown substances. The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions. Common Salts Given For Salt Analysis.
From www.youtube.com
Chemistry Uses of common salt (NaCl) Acids, bases and salts Part Common Salts Given For Salt Analysis Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. We + dil h2so4 (or dil acetic acid); Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions and cations and identification of the same in. Common Salts Given For Salt Analysis.
From www.youtube.com
Types of Salts Normal or Neutral Salts and Acidic Salts Chemistry Class Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. This experiment involves identifying the cations and anions in various salt solutions. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions. Common Salts Given For Salt Analysis.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. This experiment involves identifying the cations and anions in various salt solutions. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. The segregation of different anions and cations and identification. Common Salts Given For Salt Analysis.
From www.youtube.com
How to write salt analysis Ammonium ChlorideNH₄⁺ Cl Class 11 Common Salts Given For Salt Analysis We + dil h2so4 (or dil acetic acid); Chemists often have to identify the composition of unknown substances. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. The common ion effect is most. This experiment involves identifying the cations and anions in various salt solutions. Boil, then add solid. Common Salts Given For Salt Analysis.
From learnchemistrysaltform4.weebly.com
Qualitative analysis of salts Learn Chemistry Corner Common Salts Given For Salt Analysis Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. This experiment involves identifying the cations and anions in various salt solutions. The common ion effect is most. Chemists. Common Salts Given For Salt Analysis.
From www.researchgate.net
The properties of hydrated salts PCMs Download Scientific Diagram Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. The common ion effect is most. This experiment involves identifying the cations and anions in various salt solutions. We + dil h2so4 (or dil acetic acid); Qualitative salt analysis. Common Salts Given For Salt Analysis.
From www.slideshare.net
Gcse c6 science making useful salts Common Salts Given For Salt Analysis The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Boil, then add solid ki + fresh starch sol = deep blue. The segregation of. Common Salts Given For Salt Analysis.
From www.scribd.com
Chemistry Practicals salt analysis Salt (Chemistry) Precipitation Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. We + dil h2so4 (or dil acetic acid); This experiment involves identifying the cations and anions in various salt solutions. Boil, then add solid ki + fresh starch sol = deep blue. The common ion effect is most. The segregation of different anions and cations and identification of the same. Common Salts Given For Salt Analysis.
From www.slideshare.net
types and properties of salts Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Boil, then add solid ki + fresh starch sol = deep blue. The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. This experiment involves. Common Salts Given For Salt Analysis.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Salts Chemistry Revision & Tests Common Salts Given For Salt Analysis Chemists often have to identify the composition of unknown substances. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. We + dil h2so4 (or dil acetic acid); Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The common ion effect. Common Salts Given For Salt Analysis.
From spmchem.blogspot.com
SPM Chemistry A+ Salts Colour Common Salts Given For Salt Analysis Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. This experiment involves identifying the cations and anions in various salt solutions. Chemists often have to identify the composition of unknown substances. The common ion effect is most. Qualitative analysis of inorganic salts means the identification of cations and anions present in. Common Salts Given For Salt Analysis.
From www.studocu.com
SALT Analysis Eight Salts SALT ANALYSIS P a g e 1 Common Salts Given For Salt Analysis Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. We + dil h2so4 (or dil acetic acid); The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. This experiment involves identifying the cations and anions in various salt. Common Salts Given For Salt Analysis.
From www.youtube.com
Group 2 Salt Analysis Copper Sulphate salt Chemistry Practical Common Salts Given For Salt Analysis This experiment involves identifying the cations and anions in various salt solutions. Chemists often have to identify the composition of unknown substances. The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. Qualitative analysis of inorganic salts means the identification of cations and anions present in. Common Salts Given For Salt Analysis.
From www.thinkswap.com
Qualitative Analysis of Salts Chemistry Form 5 SPM Thinkswap Common Salts Given For Salt Analysis The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present in an unknown salt sample. The segregation of different anions and cations and identification of the same in inorganic salts is known as salt analysis. Boil, then add solid ki + fresh starch sol = deep blue. Chemists often have to. Common Salts Given For Salt Analysis.
From www.youtube.com
Salt Analysis Chemistry Practical for class 12 YouTube Common Salts Given For Salt Analysis Boil, then add solid ki + fresh starch sol = deep blue. Qualitative analysis of inorganic salts means the identification of cations and anions present in the salt or a mixture of salts. We + dil h2so4 (or dil acetic acid); The common ion effect is most. Qualitative salt analysis is the process of determining the number of constituents present. Common Salts Given For Salt Analysis.