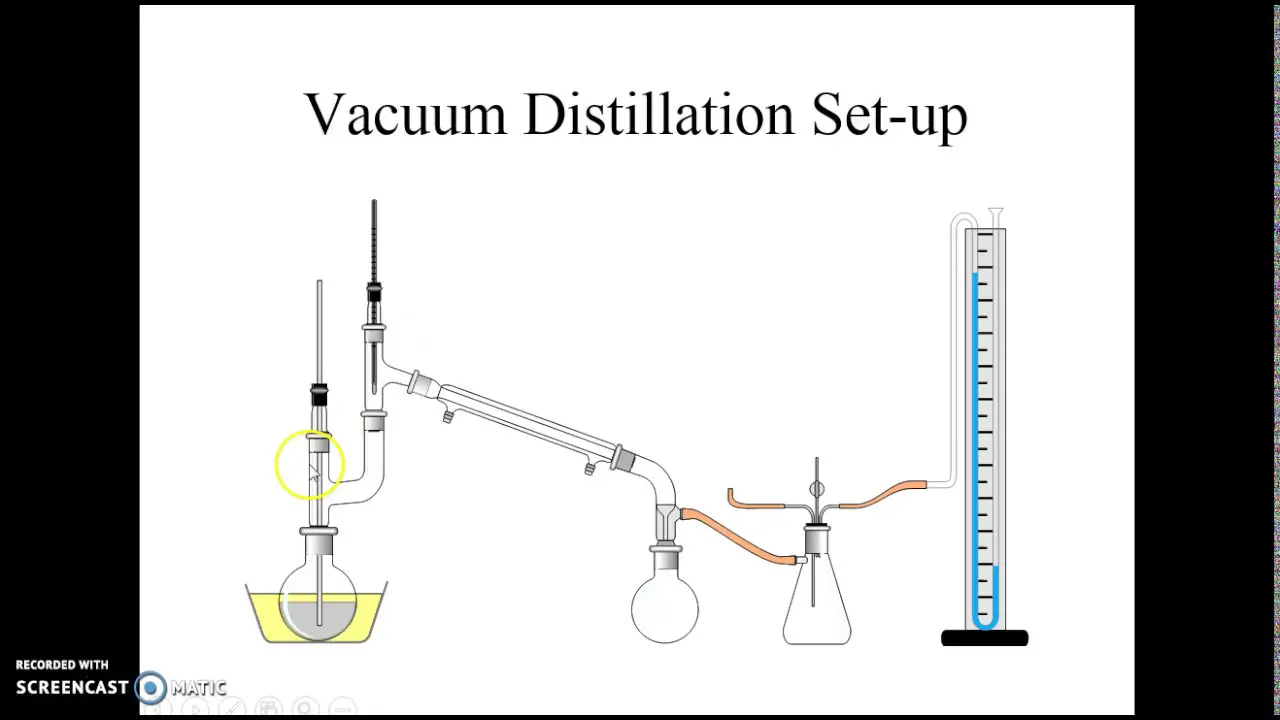
Process Of Vacuum Distillation . The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Find out how to prepare the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. By reducing their boiling point. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the.
from cleaningbeasts.com
Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. By reducing their boiling point. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Find out how to prepare the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup.
Why is Vacuum Distillation Used? Cleaning Beasts
Process Of Vacuum Distillation Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Find out how to prepare the. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. By reducing their boiling point.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! Process Of Vacuum Distillation The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. By reducing their boiling point. Find out how to prepare the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Vacuum distillation is a procedure. Process Of Vacuum Distillation.
From www.fimitech.com.tw
【Product】Vacuum distillation Taiwan Fimitech Corp. Process Of Vacuum Distillation Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the.. Process Of Vacuum Distillation.
From www.researchgate.net
Flow diagram of a dry vacuum distillation unit [9] Download Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process.. Process Of Vacuum Distillation.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Process Of Vacuum Distillation Find out how to prepare the. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Vacuum distillation is the ideal separation method. Process Of Vacuum Distillation.
From www.mdpi.com
Processes Free FullText Optimization Study on Enhancing DeepCut Process Of Vacuum Distillation Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a. Process Of Vacuum Distillation.
From onlinelibrary.wiley.com
Simulation of vacuum distillation to produce alcohol‐free beer Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Find out how. Process Of Vacuum Distillation.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Process Of Vacuum Distillation The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Find out how to prepare the. By reducing their boiling point. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Learn how to use vacuum. Process Of Vacuum Distillation.
From www.researchgate.net
Schematic diagram of vacuum membrane distillation set up Download Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Find out how to prepare the. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal. Process Of Vacuum Distillation.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Process Of Vacuum Distillation Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Find out how to prepare the. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. By reducing their boiling point. Vacuum. Process Of Vacuum Distillation.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Process Of Vacuum Distillation Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Find out how to prepare the. By reducing their boiling point. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Vacuum. Process Of Vacuum Distillation.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form Process Of Vacuum Distillation By reducing their boiling point. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Learn how to perform a vacuum distillation of a liquid. Process Of Vacuum Distillation.
From thepetrosolutions.com
Vacuum Distillation Unit in Oil Refinery The Petro Solutions Process Of Vacuum Distillation Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process.. Process Of Vacuum Distillation.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. By reducing their boiling point. Find out how to prepare the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Vacuum distillation is a. Process Of Vacuum Distillation.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes Process Of Vacuum Distillation Find out how to prepare the. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. The section also outlines safety precautions and monitoring techniques to ensure an efficient. Process Of Vacuum Distillation.
From www.e-education.psu.edu
Atmospheric and Vacuum Distillation Units FSC 432 Petroleum Refining Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process.. Process Of Vacuum Distillation.
From www.vecteezy.com
Distillation, distillation under reduced pressure, vacuum distillation Process Of Vacuum Distillation Find out how to prepare the. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. By. Process Of Vacuum Distillation.
From onlinelibrary.wiley.com
Simulation of vacuum distillation to produce alcohol‐free beer Process Of Vacuum Distillation Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when. Process Of Vacuum Distillation.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download Process Of Vacuum Distillation The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. By reducing their boiling point. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to prepare the. Vacuum. Process Of Vacuum Distillation.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to prepare the. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Vacuum distillation is a procedure or. Process Of Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Process Of Vacuum Distillation By reducing their boiling point. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Find out how to prepare the. Vacuum. Process Of Vacuum Distillation.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. Process Of Vacuum Distillation The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Learn how to use. Process Of Vacuum Distillation.
From www.alamy.com
Vacuum Distillation System Stock Vector Image & Art Alamy Process Of Vacuum Distillation Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Find out how to prepare the. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding. Process Of Vacuum Distillation.
From www.researchgate.net
A PartialVacuum Distillation Process Diagram. fluid (1) is Process Of Vacuum Distillation Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. By reducing their boiling point. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Find out how to prepare the. Learn how to perform a vacuum distillation of a. Process Of Vacuum Distillation.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' Process Of Vacuum Distillation Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. The section also outlines safety precautions and. Process Of Vacuum Distillation.
From cleaningbeasts.com
Why is Vacuum Distillation Used? Cleaning Beasts Process Of Vacuum Distillation Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Vacuum distillation is a procedure or strategy of separating a blend of compounds at. Process Of Vacuum Distillation.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding. Process Of Vacuum Distillation.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download Process Of Vacuum Distillation By reducing their boiling point. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. The section also outlines safety precautions. Process Of Vacuum Distillation.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Process Of Vacuum Distillation Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Find out how to prepare the. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. By reducing their boiling point. Learn how to perform. Process Of Vacuum Distillation.
From www.researchgate.net
Diagram of the vacuum distillation still used for purification Process Of Vacuum Distillation The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Find out how to prepare the. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the. Process Of Vacuum Distillation.
From www.processingmagazine.com
Controlling chemical vacuum processes with direct sealing diaphragm Process Of Vacuum Distillation By reducing their boiling point. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Find out how to prepare the. Learn how to use. Process Of Vacuum Distillation.
From chemicalengineeringworld.com
Types of Distillation Chemical Engineering World Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Operating a vacuum distillation column. Process Of Vacuum Distillation.
From www.researchgate.net
The schematic for the vacuum distillation process. Download Process Of Vacuum Distillation Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal atmospheric pressure. Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Find out how to prepare the. By. Process Of Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Process Of Vacuum Distillation Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. By reducing their boiling point. Find out how to prepare the. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding the. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that. Process Of Vacuum Distillation.
From www.researchgate.net
Fig. Atmosphere and Vacuum Distillation Download Scientific Diagram Process Of Vacuum Distillation Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °c) and for components in the mixture that decompose when heated under. Learn how to use vacuum distillation to separate compounds with different boiling points under reduced pressure. Operating a vacuum distillation column allows the process to occur at lower temperatures, thereby avoiding. Process Of Vacuum Distillation.
From www.buschvacuum.com
Vacuum Distillation Busch India Process Of Vacuum Distillation The section also outlines safety precautions and monitoring techniques to ensure an efficient distillation process. Find out how to prepare the. Learn how to perform a vacuum distillation of a liquid sample using a simple or fractional setup. Vacuum distillation is a procedure or strategy of separating a blend of compounds at a pressure that is lower than the normal. Process Of Vacuum Distillation.