Discussion For Titration Of Citric Acid In Juice . The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. This is a fairly standard titration except for the following: The principle acid found in orange juice is citric acid. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. To be accurate, the exact concentration of the naoh solution you prepare must be. Titration of citric acid in juice: In the determination of total acidity all the acid is assumed to be citric, so that the result. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. Titrating citric acid against strong base. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator).
from www.semanticscholar.org
Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. Titration of citric acid in juice: In the determination of total acidity all the acid is assumed to be citric, so that the result. To be accurate, the exact concentration of the naoh solution you prepare must be. Titrating citric acid against strong base. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. The principle acid found in orange juice is citric acid. This is a fairly standard titration except for the following:
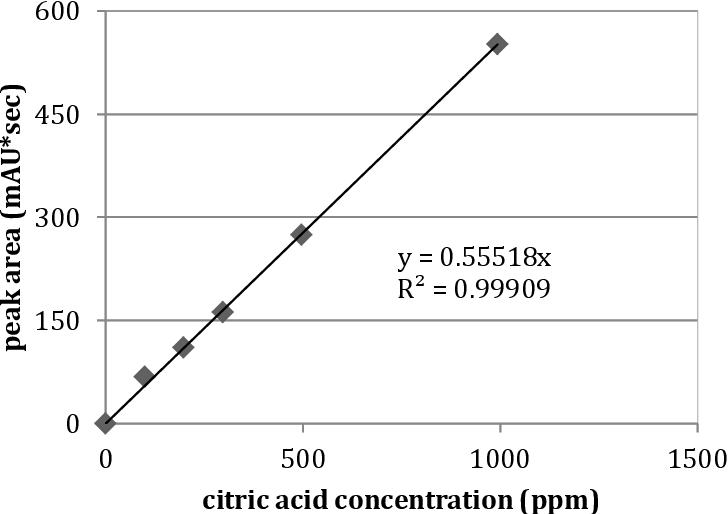
Figure 1 from Determination of citric acid in fruit juices using HPLC
Discussion For Titration Of Citric Acid In Juice The principle acid found in orange juice is citric acid. The principle acid found in orange juice is citric acid. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titrating citric acid against strong base. In the determination of total acidity all the acid is assumed to be citric, so that the result. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. Titration of citric acid in juice: This is a fairly standard titration except for the following: In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). To be accurate, the exact concentration of the naoh solution you prepare must be.
From www.semanticscholar.org
Figure 1 from Determination of citric acid in fruit juices using HPLC Discussion For Titration Of Citric Acid In Juice Titration of citric acid in juice: To be accurate, the exact concentration of the naoh solution you prepare must be. The principle acid found in orange juice is citric acid. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). This is. Discussion For Titration Of Citric Acid In Juice.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Discussion For Titration Of Citric Acid In Juice In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titration of citric acid in juice: The principle acid found in orange juice is citric acid. To be accurate, the exact concentration of. Discussion For Titration Of Citric Acid In Juice.
From www.thinkswap.com
Citric Acid Titration Practical Chemistry Year 12 SACE Thinkswap Discussion For Titration Of Citric Acid In Juice Titrating citric acid against strong base. Titration of citric acid in juice: This is a fairly standard titration except for the following: In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved Acid Base Titration D. Titration of Citric Acid in Discussion For Titration Of Citric Acid In Juice The principle acid found in orange juice is citric acid. Titration of citric acid in juice: Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. This is a fairly standard titration except for the following: In the determination of total acidity all the acid is assumed to be citric,. Discussion For Titration Of Citric Acid In Juice.
From www.studocu.com
Determination of Citric Acid in Lemon Juice Lab Instructions Please Discussion For Titration Of Citric Acid In Juice Titration of citric acid in juice: In the determination of total acidity all the acid is assumed to be citric, so that the result. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titrating citric acid against strong base. In the case of the citric acid titration, a known amount. Discussion For Titration Of Citric Acid In Juice.
From www.scribd.com
Titration of Citric Acid CHEM 103 Lab PDF Titration Chemistry Discussion For Titration Of Citric Acid In Juice The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and. Discussion For Titration Of Citric Acid In Juice.
From www.researchgate.net
Titratable acidity content ( of citric acid) and pH in juice of yellow Discussion For Titration Of Citric Acid In Juice Titrating citric acid against strong base. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. To be accurate, the exact concentration of the naoh solution you prepare must be. This is a fairly standard titration except for the following: In the case of the citric acid titration, a known amount of. Discussion For Titration Of Citric Acid In Juice.
From www.thinkswap.com
Citric Acid Titration Depth Study Investigating Science Year 11 HSC Discussion For Titration Of Citric Acid In Juice To be accurate, the exact concentration of the naoh solution you prepare must be. The principle acid found in orange juice is citric acid. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. In the case of the citric acid titration, a known amount of orange juice is measured. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved Quantitative Analysis of the Citric Acid Content in Discussion For Titration Of Citric Acid In Juice The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. In the determination of total acidity all the acid is assumed to be citric, so that the result. Titration of citric acid in juice: This is a fairly standard titration except for the following: Citric acid has three carboxylic acid groups,. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved 9. Titration Curve for Citric Acid Clearly draw the Discussion For Titration Of Citric Acid In Juice Titrating citric acid against strong base. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. In the case of the citric acid titration, a known amount of orange juice is measured. Discussion For Titration Of Citric Acid In Juice.
From dokumen.tips
(PDF) Titration of citric acid in juice Technical Notes · MSDS Sodium Discussion For Titration Of Citric Acid In Juice Titration of citric acid in juice: To be accurate, the exact concentration of the naoh solution you prepare must be. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. The principle acid found in orange juice is citric acid. In this experiment you will use a solution of naoh to. Discussion For Titration Of Citric Acid In Juice.
From www.researchgate.net
Titration curves of citric acid solution in (a) water and (b,c) in the Discussion For Titration Of Citric Acid In Juice This is a fairly standard titration except for the following: Titration of citric acid in juice: The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titrating citric acid against strong base. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. In. Discussion For Titration Of Citric Acid In Juice.
From studylib.net
Titration of Citric Acid East Stroudsburg University Discussion For Titration Of Citric Acid In Juice Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. This is a fairly standard titration except for the following: The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titration of citric acid in juice: The principle acid found in. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved (b) Complete the following table for this titration. Discussion For Titration Of Citric Acid In Juice To be accurate, the exact concentration of the naoh solution you prepare must be. In the determination of total acidity all the acid is assumed to be citric, so that the result. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. The principle acid found in orange juice is. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved Consider the following titration of Citric Acid with Discussion For Titration Of Citric Acid In Juice The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. In the determination of total acidity all the acid is assumed to be citric, so that the result. Titration of citric acid in juice: Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a /. Discussion For Titration Of Citric Acid In Juice.
From www.numerade.com
SOLVED Citric acid occurs naturally in fruit juices and acts as an Discussion For Titration Of Citric Acid In Juice The principle acid found in orange juice is citric acid. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. In the determination of total acidity all the acid is assumed to be citric, so that the result. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
D. Titration of Citric Acid in Juice Juice Powerade Discussion For Titration Of Citric Acid In Juice Titration of citric acid in juice: Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. To be accurate, the exact concentration of the naoh solution you prepare must be. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. The. Discussion For Titration Of Citric Acid In Juice.
From www.studocu.com
Titration of Sprite Procedure 1 TITRATION OF CITRIC ACID IN SPRITE Discussion For Titration Of Citric Acid In Juice In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). Titrating citric acid against strong base. Titration of citric acid in juice: In the determination of total acidity all the acid is assumed to be citric, so that the result. The principle. Discussion For Titration Of Citric Acid In Juice.
From www.youtube.com
Experiment for the Determination of amount of citric acid in lemon Discussion For Titration Of Citric Acid In Juice Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. This is a fairly standard titration except for the following: The principle acid found in orange juice is citric acid. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. In. Discussion For Titration Of Citric Acid In Juice.
From www.studocu.com
THE Determination OF Citric ACID THE DETERMINATION OF CITRIC ACID IN Discussion For Titration Of Citric Acid In Juice In the determination of total acidity all the acid is assumed to be citric, so that the result. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titration of citric acid in juice: This is a fairly standard titration except for the following: Titrating citric acid against strong base. In. Discussion For Titration Of Citric Acid In Juice.
From www.researchgate.net
(PDF) Determination of Citric acid in Soft drinks, Juice drinks and Discussion For Titration Of Citric Acid In Juice In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). To be accurate, the exact concentration of the naoh solution you prepare must be. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice.. Discussion For Titration Of Citric Acid In Juice.
From www.yumpu.com
Determination of Citric Acid in Fruit Juice Faculty web pages Discussion For Titration Of Citric Acid In Juice In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. Titrating citric acid against strong base. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. This is a fairly standard titration except for the following: The neutralization reaction with sodium hydroxide. Discussion For Titration Of Citric Acid In Juice.
From www.coursehero.com
[Solved] 2 17 points The graph below shows the titration of citric acid Discussion For Titration Of Citric Acid In Juice In the determination of total acidity all the acid is assumed to be citric, so that the result. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. The principle acid found in orange juice is citric acid. In this experiment you will use a solution of naoh to titrate. Discussion For Titration Of Citric Acid In Juice.
From www.scribd.com
Titration of Citric Acid in Juice Teacher Notes Overview/Introduction Discussion For Titration Of Citric Acid In Juice This is a fairly standard titration except for the following: In the determination of total acidity all the acid is assumed to be citric, so that the result. The principle acid found in orange juice is citric acid. Titration of citric acid in juice: Titrating citric acid against strong base. Citric acid has three carboxylic acid groups, three ionizable, acidic. Discussion For Titration Of Citric Acid In Juice.
From www.studocu.com
Experiment 4 Citric Acid inFruit Juices Background A titration is a Discussion For Titration Of Citric Acid In Juice The principle acid found in orange juice is citric acid. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). In the. Discussion For Titration Of Citric Acid In Juice.
From www.researchgate.net
(PDF) Quantitative Assessment of Juice Content, Citric Acid and Sugar Discussion For Titration Of Citric Acid In Juice To be accurate, the exact concentration of the naoh solution you prepare must be. In the determination of total acidity all the acid is assumed to be citric, so that the result. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator).. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved (b) Complete the following table for this titration. Discussion For Titration Of Citric Acid In Juice The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. Titration of citric acid in juice: In the determination of total acidity all the acid is assumed to be citric, so that the result. In this experiment you will use a solution of naoh to titrate the acid in a fruit. Discussion For Titration Of Citric Acid In Juice.
From www.thinkswap.com
Citric Juice Titration Prac Chemistry Year 11 SACE Thinkswap Discussion For Titration Of Citric Acid In Juice Titrating citric acid against strong base. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. Titration of citric acid in juice: This is a fairly standard titration except for the following: In this experiment you will use a solution of naoh to titrate the acid in a fruit juice.. Discussion For Titration Of Citric Acid In Juice.
From www.researchgate.net
(PDF) Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice Discussion For Titration Of Citric Acid In Juice The principle acid found in orange juice is citric acid. To be accurate, the exact concentration of the naoh solution you prepare must be. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. Titrating citric acid against strong base. Titration of citric acid in juice: In the determination of. Discussion For Titration Of Citric Acid In Juice.
From www.studocu.com
Citric acid Estimation by Titration method ESTIMATION OF CITRIC ACID Discussion For Titration Of Citric Acid In Juice Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three k a / pk a values. To be accurate, the exact concentration of the naoh solution you prepare must be. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation. In the determination of total acidity all. Discussion For Titration Of Citric Acid In Juice.
From www.researchgate.net
(PDF) Quantitative analysis of juice, citric acid, vitamin C content Discussion For Titration Of Citric Acid In Juice In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. Citric acid has three carboxylic acid groups, three ionizable, acidic hydrogen atoms and three. Discussion For Titration Of Citric Acid In Juice.
From www.academia.edu
(PDF) Determination of Citric acid in Soft drinks, Juice drinks and Discussion For Titration Of Citric Acid In Juice In the determination of total acidity all the acid is assumed to be citric, so that the result. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. Titration of citric acid in juice: The principle acid found in orange juice is citric acid. Titrating citric acid against strong base. To be. Discussion For Titration Of Citric Acid In Juice.
From www.chegg.com
Solved (b) the following table for this Discussion For Titration Of Citric Acid In Juice In the determination of total acidity all the acid is assumed to be citric, so that the result. In this experiment you will use a solution of naoh to titrate the acid in a fruit juice. This is a fairly standard titration except for the following: The principle acid found in orange juice is citric acid. To be accurate, the. Discussion For Titration Of Citric Acid In Juice.
From www.semanticscholar.org
Determination of Citric acid in Soft drinks, Juice drinks and Energy Discussion For Titration Of Citric Acid In Juice To be accurate, the exact concentration of the naoh solution you prepare must be. This is a fairly standard titration except for the following: Titrating citric acid against strong base. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). In the. Discussion For Titration Of Citric Acid In Juice.
From uwaterloo.ca
Acidbase titrations with citric acid part 1 Chem 13 News Magazine Discussion For Titration Of Citric Acid In Juice To be accurate, the exact concentration of the naoh solution you prepare must be. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). In the determination of total acidity all the acid is assumed to be citric, so that the result.. Discussion For Titration Of Citric Acid In Juice.