Titration Of Hydrogen Peroxide With Potassium Permanganate . The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an oxidizing agent, oxidizing. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: Titration of h2o2 with potassium permanganate. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. What you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate.
from www.chegg.com
Potassium permanganate acts as an oxidizing agent, oxidizing. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. What you need to know. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Titration of h2o2 with potassium permanganate. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide in a diluted portion of. The reaction is carried out in a. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide.
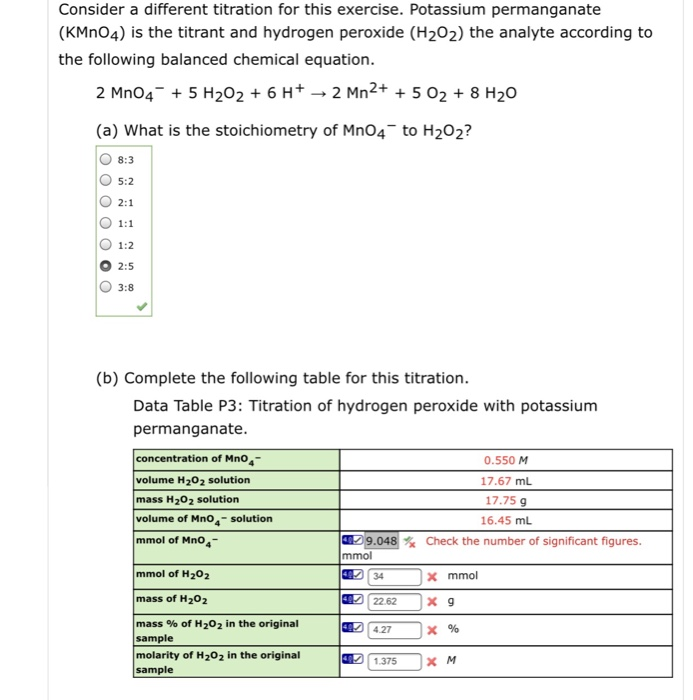
Solved Consider a different titration for this exercise.
Titration Of Hydrogen Peroxide With Potassium Permanganate To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Titration of h2o2 with potassium permanganate. What you need to know. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing. The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide.
From www.numerade.com
SOLVEDGive reactions for the oxidation of hydrogen peroxide with Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.instructables.com
of Hydrogen Peroxide by Potassium Permanganate Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: The reaction is carried out in a. Hydrogen. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Reaction equation for the titration of hydrogen Titration Of Hydrogen Peroxide With Potassium Permanganate The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Titration of h2o2 with potassium permanganate. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Find the Concentration of Hydrogen Peroxide by Titration with Potassium Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: What you need to know. The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved Titration of Hydrogen Peroxide Obiective In this Titration Of Hydrogen Peroxide With Potassium Permanganate The reaction is carried out in a. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide (h 2 o 2) is titrated with potassium. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.numerade.com
SOLVED The concentration of hydrogen peroxide solution can be found by Titration Of Hydrogen Peroxide With Potassium Permanganate Potassium permanganate acts as an oxidizing agent, oxidizing. The reaction is carried out in a. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. What you need to know. The determination is carried out by titration with. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.numerade.com
SOLVED The concentration of hydrogen peroxide in an antiseptic Titration Of Hydrogen Peroxide With Potassium Permanganate To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. What you need to know. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The reaction is carried out in a. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.reddit.com
Struggling AP student here using a titration with potassium Titration Of Hydrogen Peroxide With Potassium Permanganate Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Titration of h2o2 with potassium permanganate. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. This method describes the determination. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Potassium permanganate and hydrogen peroxide experiment YouTube Titration Of Hydrogen Peroxide With Potassium Permanganate Potassium permanganate acts as an oxidizing agent, oxidizing. What you need to know. The reaction is carried out in a. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Hydrogen peroxide in a diluted portion of. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved THE TITRATION OF AN OVERTHECOUNTER HYDROGEN Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. What you need to know. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide in a diluted portion of. Titration of h2o2 with potassium permanganate. The determination is carried out by titration. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Chemical Reaction Reaction of Potassium Permanganate With Hydrogen Titration Of Hydrogen Peroxide With Potassium Permanganate The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Potassium permanganate acts as an oxidizing agent, oxidizing. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide in a diluted portion of. This method describes. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.researchgate.net
A.) Chemical reaction between hydrogen peroxide and potassium iodide Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. What you need to know. Hydrogen peroxide in a diluted portion of. The determination is carried out by titration with. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.studocu.com
Hydrogen Peroxide Assay ASSAY OF HYDROGEN PEROXIDE BY. REDUCTION Titration Of Hydrogen Peroxide With Potassium Permanganate The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide in a diluted portion of. What you need to know. Potassium permanganate acts as an. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Gravimetric Titration of hydrogen peroxide with potassium permanganate Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
of Hydrogen Peroxide Dramatic Chemical Reaction with Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Invisible chemical Reaction of Potassium permanganate with Hydrogen Titration Of Hydrogen Peroxide With Potassium Permanganate The reaction is carried out in a. What you need to know. Hydrogen peroxide in a diluted portion of. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide (h 2 o 2). Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
AP Chemistry Investigation 8 Redox Titration of Hydrogen Peroxide by Titration Of Hydrogen Peroxide With Potassium Permanganate The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Titration. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.numerade.com
SOLVED The concentration of hydrogen peroxide solution can be found by Titration Of Hydrogen Peroxide With Potassium Permanganate Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. What you need to know. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Titration of h2o2 with potassium permanganate. Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an oxidizing agent, oxidizing. The determination is. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.numerade.com
SOLVEDsolution of hydrogen peroxide will be titrated to determine the Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: Titration of h2o2 with potassium permanganate. This method. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved Titration of Hydrogen Peroxide Obiective In this Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The reaction is carried out in a. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Titration of h2o2 with potassium permanganate. The determination is carried out by titration with potassium permanganate in a. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Titration Of Hydrogen Peroxide With Potassium Permanganate Potassium permanganate acts as an oxidizing agent, oxidizing. Titration of h2o2 with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide in a diluted portion of. What you need. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved Reaction equation for the titration of hydrogen Titration Of Hydrogen Peroxide With Potassium Permanganate Hydrogen peroxide in a diluted portion of. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The reaction is carried out in. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Hydrogen Peroxide with Potassium PermanganateTitrationRedox Reaction Titration Of Hydrogen Peroxide With Potassium Permanganate The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: Hydrogen peroxide in a diluted portion of. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The reaction is carried out in a. This method describes the determination of the concentration of hydrogen. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved Consider a different titration for this exercise. Titration Of Hydrogen Peroxide With Potassium Permanganate To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. What you need to know. The determination is carried out by titration with potassium permanganate in a. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.studypool.com
SOLUTION CHM 111 Hydrogen Peroxide by Potassium Permanganate Titration Titration Of Hydrogen Peroxide With Potassium Permanganate Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an oxidizing agent, oxidizing. Titration of h2o2 with potassium permanganate. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. What. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.scribd.com
Evonik Determination of Hydrogen Peroxide Concentration by Titration Titration Of Hydrogen Peroxide With Potassium Permanganate Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an oxidizing agent, oxidizing. Titration of h2o2 with potassium permanganate. What you need to know. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved Consider a different titration for this exercise. Titration Of Hydrogen Peroxide With Potassium Permanganate This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. The reaction is carried out in a. The determination is carried out by titration with potassium permanganate. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From studylib.net
The Potentiometric Titration of Hydrogen Peroxide Titration Of Hydrogen Peroxide With Potassium Permanganate This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: Hydrogen peroxide (h 2 o 2) is titrated. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From slideplayer.com
Volumetric Analysis Using titration of a solution with known Titration Of Hydrogen Peroxide With Potassium Permanganate Potassium permanganate acts as an oxidizing agent, oxidizing. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.youtube.com
Potassium Permanganate + Hydrogen Peroxide YouTube Titration Of Hydrogen Peroxide With Potassium Permanganate What you need to know. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.slideshare.net
Slide Titration Of Hydrogen Peroxide With Potassium Permanganate The reaction is carried out in a. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Potassium permanganate acts as an oxidizing agent, oxidizing. The determination is carried out by titration with potassium permanganate. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.chegg.com
Solved The concentration of hydrogen peroxide in a solution Titration Of Hydrogen Peroxide With Potassium Permanganate To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. Titration of h2o2 with potassium permanganate. The reaction is carried out in a. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium Titration Of Hydrogen Peroxide With Potassium Permanganate Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.slideshare.net
Potassium permanganate titrations Titration Of Hydrogen Peroxide With Potassium Permanganate Titration of h2o2 with potassium permanganate. The reaction is carried out in a. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an oxidizing agent, oxidizing. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate.. Titration Of Hydrogen Peroxide With Potassium Permanganate.
From www.studypool.com
SOLUTION CHM 111 Hydrogen Peroxide by Potassium Permanganate Titration Titration Of Hydrogen Peroxide With Potassium Permanganate The reaction is carried out in a. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Hydrogen peroxide in a diluted portion of. Titration of h2o2 with potassium permanganate. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation: To determine the concentration of. Titration Of Hydrogen Peroxide With Potassium Permanganate.