Standard Heat Enthalpy . the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. The elemental form of each atom is that. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
from www.studocu.com
a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The elemental form of each atom is that. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.
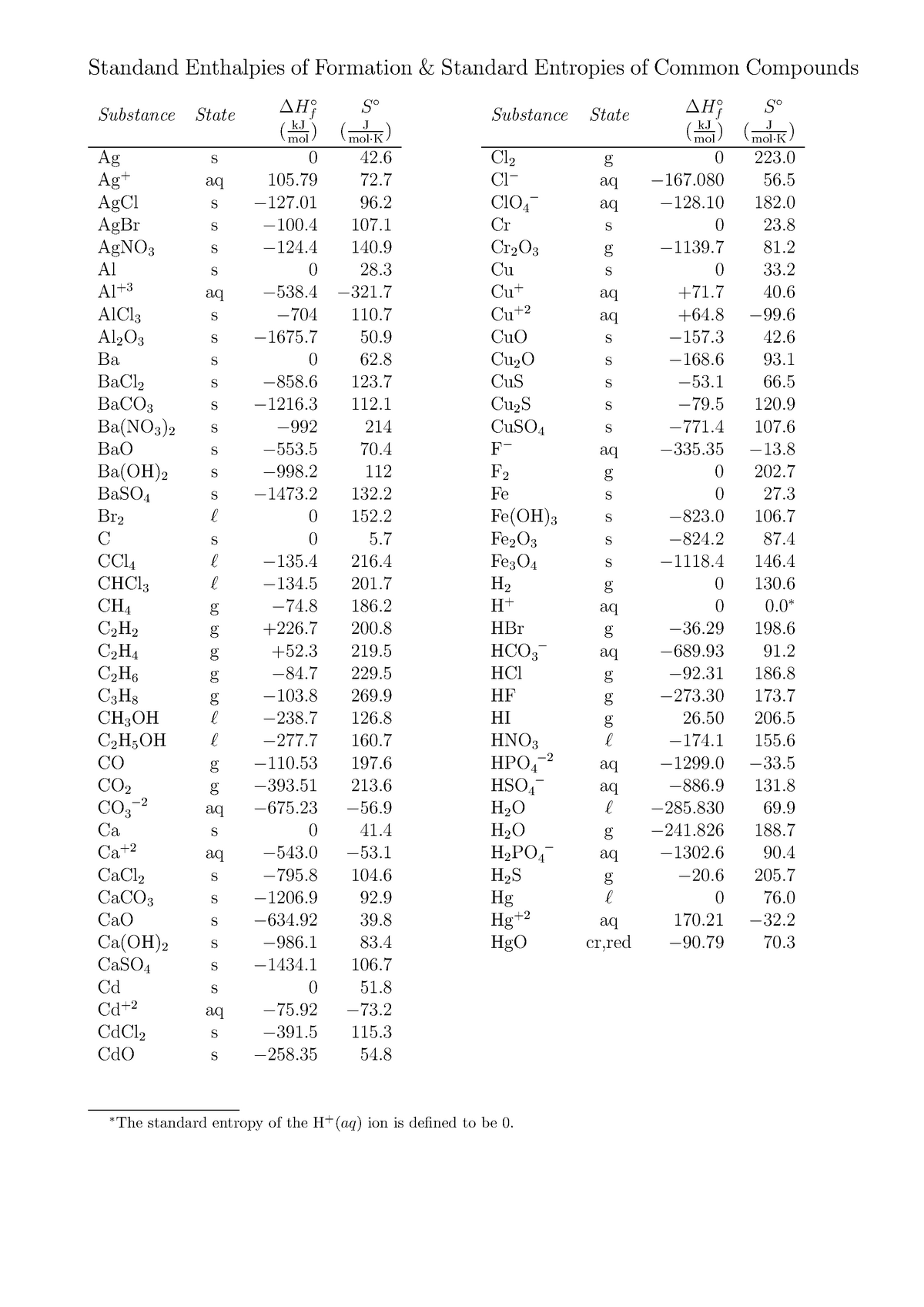
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol
Standard Heat Enthalpy a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The elemental form of each atom is that. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1. Standard Heat Enthalpy.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (denoted ). Standard Heat Enthalpy.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Enthalpy a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136. Standard Heat Enthalpy.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Enthalpy 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of. Standard Heat Enthalpy.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Heat Enthalpy enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Heat Enthalpy.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Heat Enthalpy 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The elemental form of each atom is that. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature. Standard Heat Enthalpy.
From www.nagwa.com
Question Video Calculating the the Enthalpy Change for the Thermal Standard Heat Enthalpy enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total. Standard Heat Enthalpy.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Heat Enthalpy The elemental form of each atom is that. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard. Standard Heat Enthalpy.
From dbdalrympleshapably.z21.web.core.windows.net
How To Calculate The Enthalpy Standard Heat Enthalpy the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Heat Enthalpy.
From dbdalrympleshapably.z21.web.core.windows.net
How To Calculate The Enthalpy Standard Heat Enthalpy The elemental form of each atom is that. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product. Standard Heat Enthalpy.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Heat Enthalpy the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables. Standard Heat Enthalpy.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant.. Standard Heat Enthalpy.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Standard Heat Enthalpy enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Heat Enthalpy.
From www.chemistrylearner.com
Heat (Enthalpy) of Neutralization Definition and Formula Standard Heat Enthalpy enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a.. Standard Heat Enthalpy.
From schoolworkhelper.net
StandardEnthalpiesFormation Standard Heat Enthalpy a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1. Standard Heat Enthalpy.
From www.vrogue.co
Enthalpy Change Of Combustion Ppt Enthalpy Of Formati vrogue.co Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a.. Standard Heat Enthalpy.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 Standard Heat Enthalpy the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm.. Standard Heat Enthalpy.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Heat Enthalpy enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and. Standard Heat Enthalpy.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. . Standard Heat Enthalpy.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. . Standard Heat Enthalpy.
From www.researchgate.net
Enthalpy of formation and heats of formation of main combustion Standard Heat Enthalpy The elemental form of each atom is that. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The standard state for measuring and reporting enthalpies. Standard Heat Enthalpy.
From learningschoollitahet.z22.web.core.windows.net
Enthalpy Calculation Worksheet Standard Heat Enthalpy The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of. Standard Heat Enthalpy.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Heat Enthalpy a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole. Standard Heat Enthalpy.
From lessonlibrarystiletto.z13.web.core.windows.net
Heat Of Reaction Equation Standard Heat Enthalpy the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The elemental form of each atom is that. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. 193 rows in chemistry and thermodynamics, the standard. Standard Heat Enthalpy.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. the. Standard Heat Enthalpy.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a. Standard Heat Enthalpy.
From printablelistquinta.z21.web.core.windows.net
Explain Heat Of Formation Standard Heat Enthalpy 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. the standard enthalpy of reaction. Standard Heat Enthalpy.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Standard Heat Enthalpy the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. enthalpies and enthalpy changes. Standard Heat Enthalpy.
From learningzonevygiekt.z13.web.core.windows.net
Heat Of Formation Formula Standard Heat Enthalpy The elemental form of each atom is that. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. 193 rows in chemistry and thermodynamics, the. Standard Heat Enthalpy.
From marielatinrobertson.blogspot.com
Standard Enthalpy of Combustion MarielatinRobertson Standard Heat Enthalpy a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The elemental form of each atom is that. the standard enthalpy of reaction (denoted ) for a chemical. Standard Heat Enthalpy.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Heat Enthalpy the standard enthalpy of reaction (denoted ) for a chemical reaction is the difference between total product and total reactant. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1. Standard Heat Enthalpy.
From jamesherbert.z13.web.core.windows.net
Heat Of Formation Chart Standard Heat Enthalpy the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. enthalpies and enthalpy changes for reactions vary as a function of temperature, [5] but tables generally list the standard. the standard enthalpy of reaction. Standard Heat Enthalpy.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Heat Enthalpy 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The elemental form of each atom. Standard Heat Enthalpy.
From hxelasqby.blob.core.windows.net
Standard Heat Of Reaction Using Enthalpies Of Formation at Phyllis Standard Heat Enthalpy 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. . Standard Heat Enthalpy.
From dxorvtvqp.blob.core.windows.net
Standard Enthalpy Of Formation Gibbsite at Samuel Speed blog Standard Heat Enthalpy a standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. The elemental form of each atom is that. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. the standard enthalpy of reaction (denoted ) for a chemical reaction. Standard Heat Enthalpy.