Buffer Example Biology . The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Protein buffer systems work predominantly inside cells. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. This is simply because most life on. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). Biological buffers are organic substances that help regulate the ph level in organisms. Buffers in biology and biological buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. In this way, a biological buffer. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
from studylib.net
They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. Protein buffer systems work predominantly inside cells. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are organic substances that help regulate the ph level in organisms. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). Buffers in biology and biological buffers.
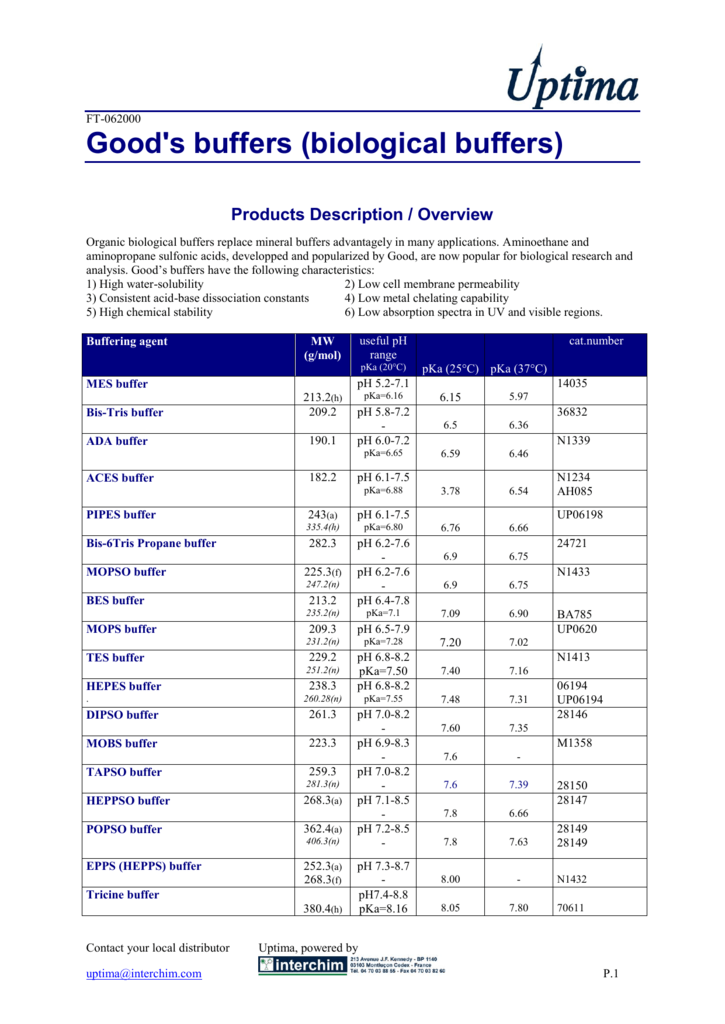
Good's buffers (biological buffers)
Buffer Example Biology Buffers in biology and biological buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. Protein buffer systems work predominantly inside cells. Buffers in biology and biological buffers. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. This is simply because most life on. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Buffer Example Biology Biological buffers are organic substances that help regulate the ph level in organisms. Buffers in biology and biological buffers. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. In this. Buffer Example Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Example Biology This is simply because most life on. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Buffers in biology and biological buffers. The purpose of a buffer. Buffer Example Biology.
From www.brainkart.com
Buffers Buffer Example Biology Protein buffer systems work predominantly inside cells. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers in biology and biological buffers. The most relevant systems for biology are the carbonic. Buffer Example Biology.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination For Biologists Buffer Example Biology Biological buffers are organic substances that help regulate the ph level in organisms. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). The most relevant systems for biology are the carbonic. Buffer Example Biology.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Buffer Example Biology The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. In this way, a biological buffer. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. A biological buffer is an. Buffer Example Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Example Biology Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. This is simply because most life on. Buffers in biology and biological buffers. A biological buffer is an organic substance that has. Buffer Example Biology.
From www.youtube.com
Role of buffers in biological system YouTube Buffer Example Biology Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Buffer Example Biology.
From studylib.net
Good's buffers (biological buffers) Buffer Example Biology The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and. Buffer Example Biology.
From www.youtube.com
Buffers and pH Biology YouTube Buffer Example Biology Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The purpose of a buffer in a. Buffer Example Biology.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Buffer Example Biology In this way, a biological buffer. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. This is simply because most life on. Buffers in biology and biological buffers. Protein buffer systems work predominantly inside cells. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer Example Biology.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Buffer Example Biology Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. Buffers in biology and biological buffers. This is simply because most life on. The most relevant systems for biology are the carbonic. Buffer Example Biology.
From www.researchgate.net
Examples of buffer compounds divided into accepted physiological Buffer Example Biology This is simply because most life on. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological systems have peak activity in a very ph narrow range (at a ph. Buffer Example Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Buffer Example Biology This is simply because most life on. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). In this way, a biological buffer. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. In this article, you will be able to describe. Buffer Example Biology.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards Buffer Example Biology Buffers in biology and biological buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. This is simply because most life on. Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. The purpose of a buffer in a biological system is to. Buffer Example Biology.
From www.slideserve.com
PPT Chapter 21 “Neutralization” PowerPoint Presentation, free Buffer Example Biology This is simply because most life on. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. In this article, you will be able to describe what a buffer is, why buffers are important,. Buffer Example Biology.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffer Example Biology Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a. Buffer Example Biology.
From mavink.com
Acids Bases And Buffers Buffer Example Biology In this way, a biological buffer. Buffers in biology and biological buffers. Biological buffers are organic substances that help regulate the ph level in organisms. This is simply because most life on. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. A biological buffer is an organic substance. Buffer Example Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Example Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological buffers are organic substances that help regulate the ph level in organisms. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. In this article, you will be able to describe what a. Buffer Example Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Buffer Example Biology In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. Biological buffers are organic substances that help regulate the ph level in organisms. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. The. Buffer Example Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Example Biology Buffers in biology and biological buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. Biological buffers are organic substances that help regulate the ph level in organisms. Protein buffer systems work predominantly inside cells. In this way, a biological buffer. The purpose of. Buffer Example Biology.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Buffer Example Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. This is simply because most life on. Biological buffers are organic substances that help regulate the ph level in organisms. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in.. Buffer Example Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Example Biology Buffers in biology and biological buffers. Protein buffer systems work predominantly inside cells. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. In this way, a biological buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer Example Biology.
From saylordotorg.github.io
Buffers Buffer Example Biology In this way, a biological buffer. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). Protein buffer systems work predominantly inside cells. This is simply because most life on. The most relevant systems. Buffer Example Biology.
From www.youtube.com
pH and buffers overview for biology YouTube Buffer Example Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. Protein buffer systems work predominantly inside cells. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. In this way, a biological buffer. They. Buffer Example Biology.
From www.slideshare.net
Buffer system Buffer Example Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological buffers are organic substances that help regulate the ph level in organisms. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. In this way, a biological buffer. Biological. Buffer Example Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Buffer Example Biology The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological buffer. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph. Buffer Example Biology.
From www.slideshare.net
Buffer system Buffer Example Biology Buffers in biology and biological buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Protein buffer systems work predominantly inside cells. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). This is. Buffer Example Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Example Biology This is simply because most life on. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range.. Buffer Example Biology.
From www.youtube.com
WCLN Buffers Biology YouTube Buffer Example Biology This is simply because most life on. Protein buffer systems work predominantly inside cells. Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. The buffer. Buffer Example Biology.
From studylib.net
Buffer Solutions Buffer Example Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and the phosphate. Buffers in biology and biological buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. The purpose of a buffer in a biological. Buffer Example Biology.
From www.youtube.com
Buffer action in the blood YouTube Buffer Example Biology The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Protein buffer systems work predominantly inside cells. Biological buffers are organic substances that help regulate the ph level in organisms. The. Buffer Example Biology.
From www.expii.com
Buffers — Definition & Overview Expii Buffer Example Biology The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological significance in. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Buffer Example Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Example Biology Buffers in biology and biological buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). This is simply because most life on. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The most relevant systems for biology are the carbonic. Buffer Example Biology.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Example Biology This is simply because most life on. They act by neutralizing excess hydrogen ions, thereby maintaining the ph within a narrow and optimal range. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological buffer. Biological buffers are organic substances that help regulate the ph level in organisms.. Buffer Example Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Buffer Example Biology In this way, a biological buffer. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. This is simply because most life on. Protein buffer systems work predominantly inside cells. In this article, you will be able to describe what a buffer is, why buffers are important, and how specific buffers have biological. Buffer Example Biology.