Specific Heat Of Combustion Gases . The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat and depends on the material and phase. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. standard heat of combustion : the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines.
from www.animalia-life.club
standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat and depends on the material and phase. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. The energy liberated when a substance x undergoes complete combustion, with excess of. standard heat of combustion : 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned.
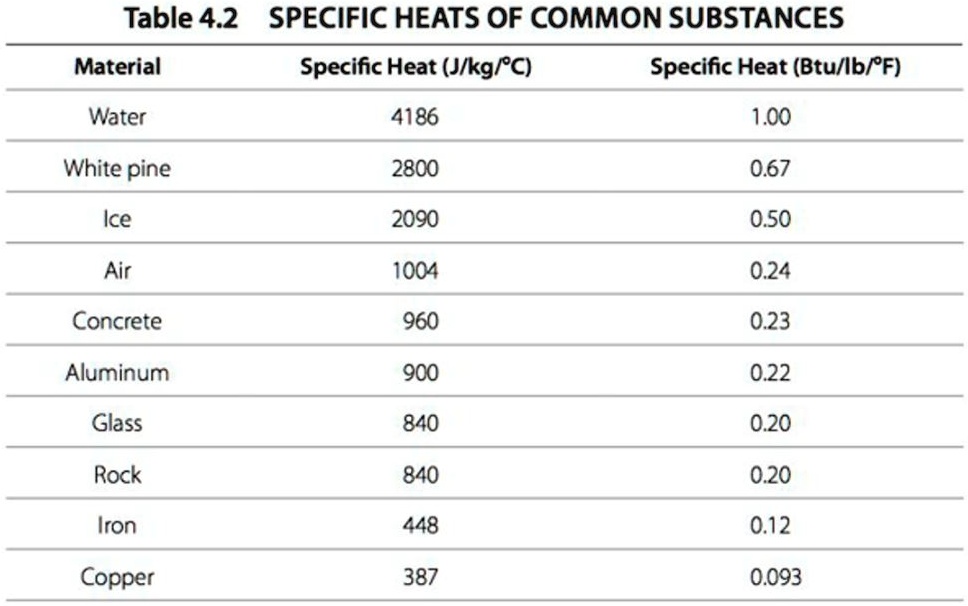
Specific Heat Chart Of Common Substances
Specific Heat Of Combustion Gases the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The energy liberated when a substance x undergoes complete combustion, with excess of. The specific heat is the amount of heat necessary to change the temperature. standard heat of combustion : the symbol c stands for specific heat and depends on the material and phase. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,.
From solvedlib.com
Consider an ideal gasturbine cycle with two stages o… SolvedLib Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the symbol c stands for specific heat and depends on the material and phase. The energy liberated when a substance x undergoes complete combustion, with excess of. the molar heat of combustion \(\left( he \right)\). Specific Heat Of Combustion Gases.
From www.chemicals.co.uk
Examples of Combustion Reactions in Chemistry The Chemistry Blog Specific Heat Of Combustion Gases standard heat of combustion : the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the specific heat (= specific heat capacity) at. Specific Heat Of Combustion Gases.
From www.researchgate.net
Ratio of specific heats computed at different temperatures and mixture Specific Heat Of Combustion Gases the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the symbol c stands for specific heat and depends on the material and phase. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a.. Specific Heat Of Combustion Gases.
From www.askphysics.com
Why gases have 2 specific heats? Ask Physics Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard heat of combustion : the symbol c stands for specific heat and depends on the material and phase. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and. Specific Heat Of Combustion Gases.
From www.researchgate.net
Combustion heat and gas specific volume of highenergy expansion agent Specific Heat Of Combustion Gases standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature. the molar heat of combustion \(\left( he \right)\) is the heat released. Specific Heat Of Combustion Gases.
From www.youtube.com
3. 11P13.2 CV 2 Specific Heat Capacities of Gases and Mean Free Path Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. The specific heat is the amount of heat necessary to change the temperature. standard heat. Specific Heat Of Combustion Gases.
From www.grc.nasa.gov
enthalpy Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The energy liberated when a. Specific Heat Of Combustion Gases.
From www.researchgate.net
(PDF) An integrated sensor technology for measurements of specific heat Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The specific heat is the amount of heat necessary to change the temperature. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. the molar. Specific Heat Of Combustion Gases.
From exoikrrkt.blob.core.windows.net
Evaporation And Specific Heat at Vicki Siddiqui blog Specific Heat Of Combustion Gases standard heat of combustion : The specific heat is the amount of heat necessary to change the temperature. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. the symbol c stands for specific heat and depends on the material and phase. the specific heat (=. Specific Heat Of Combustion Gases.
From www.youtube.com
20 Specific Heats of Ideal Gases&Liquids&Solids YouTube Specific Heat Of Combustion Gases the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard heat of combustion : standard enthalpy of combustio n (\(δh_c^\circ\)) is the. Specific Heat Of Combustion Gases.
From www.grc.nasa.gov
Specific Heats Calorically Imperfect Gas Specific Heat Of Combustion Gases standard heat of combustion : the symbol c stands for specific heat and depends on the material and phase. The energy liberated when a substance x undergoes complete combustion, with excess of. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. The specific heat is. Specific Heat Of Combustion Gases.
From www.vrogue.co
Table Of Specific Heats vrogue.co Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. standard heat of combustion : The specific heat is the amount of heat necessary to change the temperature. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 84 rows by convention,. Specific Heat Of Combustion Gases.
From www.slideserve.com
PPT State Postulate PowerPoint Presentation, free download ID6647618 Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a. Specific Heat Of Combustion Gases.
From unacademy.com
Notes on Formulas Involved With the Specific Heat Capacity of Gases Specific Heat Of Combustion Gases standard heat of combustion : The specific heat is the amount of heat necessary to change the temperature. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. . Specific Heat Of Combustion Gases.
From www.youtube.com
Enthalpy of combustion hRP YouTube Specific Heat Of Combustion Gases standard heat of combustion : the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the molar heat of combustion \(\left( he \right)\) is the heat released when. Specific Heat Of Combustion Gases.
From www.conceptualphysicstoday.com
Conceptual Physics Specific Heat of Gases Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. The energy liberated when a substance x undergoes complete combustion, with excess of. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. standard heat of combustion : standard enthalpy of combustio. Specific Heat Of Combustion Gases.
From webapi.bu.edu
💐 Heat evolved during combustion. The heat evolved during the Specific Heat Of Combustion Gases the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy. Specific Heat Of Combustion Gases.
From www.slideserve.com
PPT Specific heat of gases PowerPoint Presentation, free download Specific Heat Of Combustion Gases standard heat of combustion : the symbol c stands for specific heat and depends on the material and phase. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The energy liberated when a substance x undergoes complete combustion, with excess of. the specific heat (= specific heat. Specific Heat Of Combustion Gases.
From www.researchgate.net
Fuel gas composition and combustion heat Download Table Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The specific heat is the amount of heat necessary to change the temperature. the molar heat of combustion \(\left( he \right)\). Specific Heat Of Combustion Gases.
From www.chegg.com
Solved TABLE A20 Ideal Gas Specific Heats of Some Common Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h,. Specific Heat Of Combustion Gases.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Specific Heat Of Combustion Gases standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The energy liberated when a substance x undergoes complete combustion, with excess of. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the thermal properties of a. Specific Heat Of Combustion Gases.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Specific Heat Of Combustion Gases standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the symbol c stands for specific heat and depends on the material and phase. The energy liberated. Specific Heat Of Combustion Gases.
From www.researchgate.net
Change in the specific heat of combustion of fuel with an increase in Specific Heat Of Combustion Gases The energy liberated when a substance x undergoes complete combustion, with excess of. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. standard heat of combustion : the symbol c stands for specific heat and depends on the material and phase. The specific heat is the. Specific Heat Of Combustion Gases.
From studylib.net
Table of Specific Heats Specific Heat Of Combustion Gases the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The specific heat is the amount of heat necessary to change the temperature. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows by convention, the (higher). Specific Heat Of Combustion Gases.
From heerabigyan.blogspot.com
27+ Heat Of Combustion Calculation HeeraBigyan Specific Heat Of Combustion Gases standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The specific heat is the amount of heat necessary to change the temperature. standard heat of combustion : the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned.. Specific Heat Of Combustion Gases.
From www.researchgate.net
Values of heat of gasification and effective heat of combustion Fuel L Specific Heat Of Combustion Gases standard heat of combustion : standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. The energy liberated when a substance x undergoes complete combustion, with excess of. . Specific Heat Of Combustion Gases.
From www.chegg.com
Chemistry Archive March 08, 2017 Specific Heat Of Combustion Gases 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard heat of combustion : the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. the specific heat (= specific heat capacity) at constant. Specific Heat Of Combustion Gases.
From www.youtube.com
[4.5] Heat of combustion YouTube Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The specific heat is the amount of heat necessary to change the temperature.. Specific Heat Of Combustion Gases.
From www.researchgate.net
6. Heat capacity of the flue gas depending on temperature and air Specific Heat Of Combustion Gases standard heat of combustion : standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific heat, cp,. The energy liberated when a substance x undergoes complete combustion, with excess of. . Specific Heat Of Combustion Gases.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. . Specific Heat Of Combustion Gases.
From www.slideserve.com
PPT 5 Energetics PowerPoint Presentation, free download ID3527727 Specific Heat Of Combustion Gases the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. standard heat of combustion : standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the specific heat (= specific heat capacity) at constant pressure and constant. Specific Heat Of Combustion Gases.
From www.researchgate.net
Specific heat of flue gas under various EA ratios and flue gas Specific Heat Of Combustion Gases standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the thermal properties of a pure substance are described by quantities including internal energy, u, enthalpy, h, specific. Specific Heat Of Combustion Gases.
From www.researchgate.net
Change in the specific heat of combustion of fuel with an increase in Specific Heat Of Combustion Gases standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the. Specific Heat Of Combustion Gases.
From byjus.com
Calculate standard heat of combustion of ethanol(C2H5OH(I)). Given that Specific Heat Of Combustion Gases the symbol c stands for specific heat and depends on the material and phase. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. standard heat of combustion : the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a. Specific Heat Of Combustion Gases.
From www.worldatlas.com
What Are The Properties Of Matter? WorldAtlas Specific Heat Of Combustion Gases the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. The specific heat is the amount of heat necessary to change the temperature. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. standard enthalpy of. Specific Heat Of Combustion Gases.