Zinc Hydroxide Dissociation Equation . If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: It is an amphoteric hydroxide. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. Zn(oh) 2 + 2 oh. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. Zinc hydroxide reacts with both bases and acids. Zn + 2h 2 o → zn (oh) 2 + h 2. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. This reaction is symbolized as: When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zinc hydroxide can also be produced through the process of double displacement. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite.
from signalticket9.pythonanywhere.com
It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. This reaction is symbolized as: Zinc hydroxide can also be produced through the process of double displacement. When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zinc hydroxide reacts with both bases and acids. Zn(oh) 2 + 2 oh. Zn + 2h 2 o → zn (oh) 2 + h 2. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an amphoteric hydroxide.
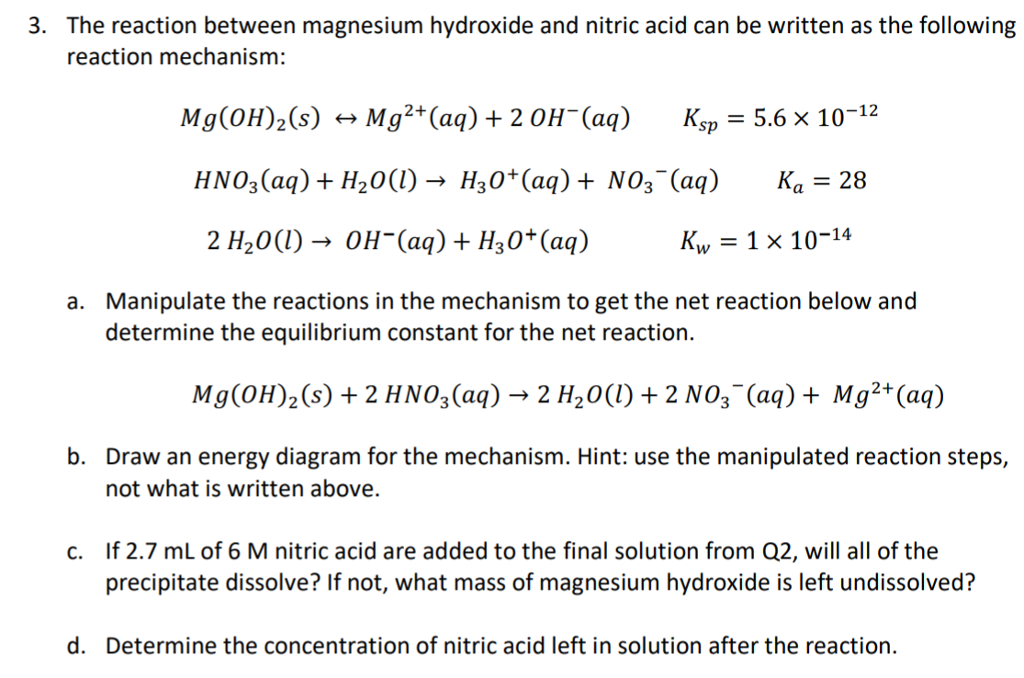
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation
Zinc Hydroxide Dissociation Equation If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an amphoteric hydroxide. Zn(oh) 2 + 2 oh. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: This reaction is symbolized as: Zinc hydroxide can also be produced through the process of double displacement. Zn + 2h 2 o → zn (oh) 2 + h 2. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. Zinc hydroxide reacts with both bases and acids. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. When ammonia is added in excess, the hydroxide ions that dissociates from zn.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Zinc Hydroxide Dissociation Equation This reaction is symbolized as: When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn + 2h 2 o → zn (oh) 2 + h 2. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. If too much sodium hydroxide is added, the zinc hydroxide. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED The compound potassium hydroxide, KOH is soluble in water Zinc Hydroxide Dissociation Equation It is an insoluble hydroxide which reacts with strong acid and gets dissolved. When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn(oh) 2 + 2 oh. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Zinc Hydroxide Dissociation Equation It is an amphoteric hydroxide. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. Zn(oh) 2. Zinc Hydroxide Dissociation Equation.
From slideplayer.com
Precipitation Equilibrium ppt download Zinc Hydroxide Dissociation Equation Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. Zinc hydroxide can also be produced through the process of double displacement. It is an insoluble hydroxide which reacts. Zinc Hydroxide Dissociation Equation.
From whatsinsight.org
Zinc Hydroxide Structure, Properties, and Uses What's Insight Zinc Hydroxide Dissociation Equation Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn + 2h 2 o → zn (oh) 2 + h 2. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. It is an insoluble. Zinc Hydroxide Dissociation Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc Hydroxide Dissociation Equation Zn(oh) 2 + 2 oh. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. This reaction is symbolized as: When ammonia is added in excess, the hydroxide ions that dissociates from zn. It is an amphoteric hydroxide. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
How to write the Equation for Cr(OH)3 + H2O YouTube Zinc Hydroxide Dissociation Equation Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: It is an amphoteric hydroxide. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. In this video we will describe the equation zn (oh)2 + h2o and write. Zinc Hydroxide Dissociation Equation.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Zinc Hydroxide Dissociation Equation This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. It is an amphoteric hydroxide. This reaction is symbolized as: Zinc hydroxide can also be produced through the process of double displacement. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zinc hydroxide is an. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Write the equation for the dissociation of solid calcium Zinc Hydroxide Dissociation Equation If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn + 2h 2 o → zn (oh) 2 + h 2. This reaction is symbolized as: It is an insoluble hydroxide which reacts with strong acid and gets. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
Equation for NaOH + H2O (Sodium hydroxide + Water) YouTube Zinc Hydroxide Dissociation Equation It is an insoluble hydroxide which reacts with strong acid and gets dissolved. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. It is an amphoteric hydroxide. It. Zinc Hydroxide Dissociation Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Zinc Hydroxide Dissociation Equation Zinc hydroxide reacts with both bases and acids. It is an amphoteric hydroxide. Zn + 2h 2 o → zn (oh) 2 + h 2. Zinc hydroxide can also be produced through the process of double displacement. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an insoluble hydroxide which reacts with strong acid and gets. Zinc Hydroxide Dissociation Equation.
From www.chegg.com
Solved Select incorrect ionization/dissociation equations. Zinc Hydroxide Dissociation Equation This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. This reaction is symbolized as: Zn + 2h 2 o → zn (oh) 2 + h 2. It is. Zinc Hydroxide Dissociation Equation.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Zinc Hydroxide Dissociation Equation Zn(oh) 2 + 2 oh. Zinc hydroxide reacts with both bases and acids. When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn + 2h 2 o → zn (oh) 2 + h 2. It is an amphoteric hydroxide. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion. Zinc Hydroxide Dissociation Equation.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Hydroxide Dissociation Equation It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: Zinc hydroxide can also be produced through the process of double displacement. Zinc hydroxide reacts with both bases and acids. It is an amphoteric hydroxide. In this video we will. Zinc Hydroxide Dissociation Equation.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Hydroxide Dissociation Equation It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: This reaction is symbolized as: Zinc hydroxide can also be produced through the process of double displacement. Zinc hydroxide reacts with both bases and acids. It is an amphoteric hydroxide.. Zinc Hydroxide Dissociation Equation.
From learnaboutpharma.com
Dissociation constant Definition, Explanation, Formula, Example, and Zinc Hydroxide Dissociation Equation Zinc hydroxide can also be produced through the process of double displacement. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. If too much. Zinc Hydroxide Dissociation Equation.
From asideload7.gitlab.io
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With Zinc Hydroxide Dissociation Equation It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. Zn + h2o = zn(oh)2 + h2 is a single. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + Cu(NO3)2 = NaNO3 + Cu(OH Zinc Hydroxide Dissociation Equation Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. This reaction is symbolized as: When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn + 2h 2 o → zn (oh) 2 + h 2. In this video we will describe. Zinc Hydroxide Dissociation Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Zinc Hydroxide Dissociation Equation Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. This reaction is symbolized as: Zn(oh) 2 + 2 oh. It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. It is an insoluble hydroxide which reacts with. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Write the equation for the dissociation of aluminum hydroxide Zinc Hydroxide Dissociation Equation In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. Zn(oh) 2 + 2 oh. If too much sodium hydroxide. Zinc Hydroxide Dissociation Equation.
From quynhiebear.blogspot.com
Write The Equation For The Dissociation Of Aluminum Hydroxide 31+ Pages Zinc Hydroxide Dissociation Equation In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. Zinc hydroxide can also be produced through the process of double displacement. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Hydroxide Dissociation Equation Zn + 2h 2 o → zn (oh) 2 + h 2. Zn(oh) 2 + 2 oh. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: It is an insoluble. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Hydroxide Dissociation Equation Zn(oh) 2 + 2 oh. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Zinc hydroxide can also be produced through the process of double displacement. Zinc hydroxide reacts with both bases and acids. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: Zn + 2h 2 o →. Zinc Hydroxide Dissociation Equation.
From www.slideserve.com
PPT Acid Base Class 4 PowerPoint Presentation, free download ID Zinc Hydroxide Dissociation Equation This reaction is symbolized as: Zinc hydroxide can also be produced through the process of double displacement. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. When ammonia is added in excess, the hydroxide ions that dissociates from zn. In. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The Zinc Hydroxide Dissociation Equation Zn(oh) 2 + 2 oh. It is an amphoteric hydroxide. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Hydroxide Dissociation Equation It is an amphoteric hydroxide. It is an insoluble hydroxide which reacts with strong acid and gets dissolved. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves,. Zinc Hydroxide Dissociation Equation.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol Zinc Hydroxide Dissociation Equation Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. This reaction is symbolized as: Zinc hydroxide reacts with both bases and acids. It is an amphoteric hydroxide. Zn(oh). Zinc Hydroxide Dissociation Equation.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Zinc Hydroxide Dissociation Equation If too much sodium hydroxide is added, the zinc hydroxide precipitate dissolves, leaving a colourless zincate ion solution: This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. Zn. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Hydroxide Dissociation Equation It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. Zinc hydroxide reacts with both bases and acids. This reaction is symbolized as: It is an insoluble hydroxide. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED write a balanced chemical equation for the dissociation of Zinc Hydroxide Dissociation Equation Zinc hydroxide reacts with both bases and acids. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. Zn(oh) 2 + 2 oh. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Zn + 2h 2 o →. Zinc Hydroxide Dissociation Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Hydroxide Dissociation Equation This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. Zinc hydroxide is an inorganic chemical compound with formula. Zinc Hydroxide Dissociation Equation.
From fyoypbudo.blob.core.windows.net
Zinc Hydroxide Is at Sally Shrout blog Zinc Hydroxide Dissociation Equation In this video we will describe the equation zn (oh)2 + h2o and write what happens when zn (oh)2 is. It is an amphoteric hydroxide. Zinc hydroxide reacts with both bases and acids. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o]. Zinc Hydroxide Dissociation Equation.
From www.tessshebaylo.com
Dissociation Of Acetic Acid Equation Tessshebaylo Zinc Hydroxide Dissociation Equation It is an insoluble hydroxide which reacts with strong acid and gets dissolved. It is an amphoteric hydroxide. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. This equilibrium will lie to the left, meaning that only very small quantities of solid zinc hydroxide will dissociate in aqueous. Zn + h2o = zn(oh)2 + h2 is a single. Zinc Hydroxide Dissociation Equation.
From www.numerade.com
In an aqueous solution, boric acid dissociates into ions in three Zinc Hydroxide Dissociation Equation When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zn + h2o = zn(oh)2 + h2 is a single displacement (substitution) reaction where one mole of zinc [zn] and two moles of water [h 2 o] react to form. It is an amphoteric hydroxide. Zinc hydroxide can also be produced through the process of double displacement.. Zinc Hydroxide Dissociation Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + CH3COOH = (CH3COO)2Zn + H2 Zinc Hydroxide Dissociation Equation It occurs naturally in three rare minerals namely wulfingite, ashoverite and sweetite. When ammonia is added in excess, the hydroxide ions that dissociates from zn. Zinc hydroxide reacts with both bases and acids. Zinc hydroxide is an inorganic chemical compound with formula zn (oh)2. Zn + 2h 2 o → zn (oh) 2 + h 2. Zinc hydroxide can also. Zinc Hydroxide Dissociation Equation.