Titration Chemical Reaction . titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn about the four types of titration: learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction.
from chem.libretexts.org
learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. learn about the four types of titration: titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos.
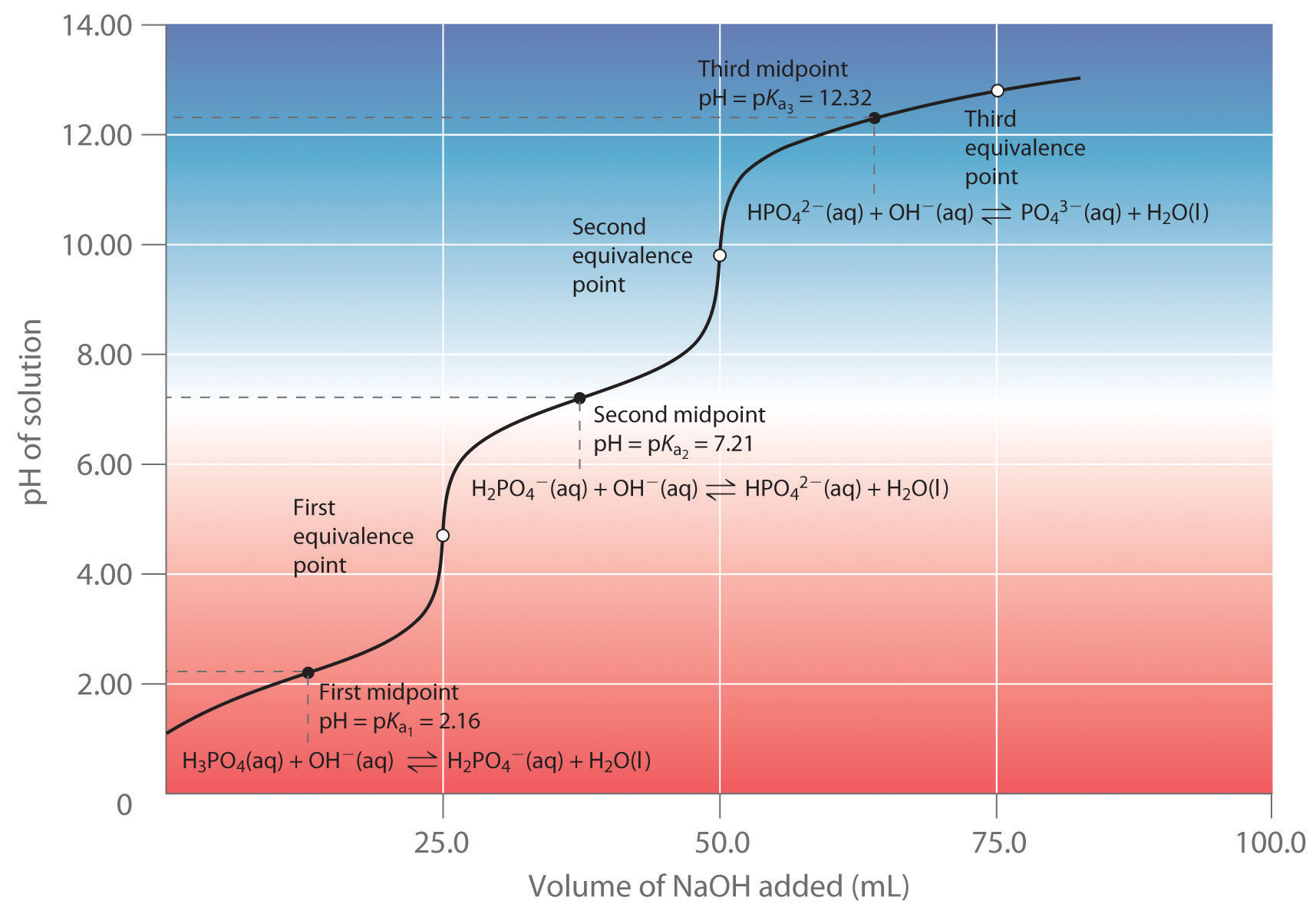
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts
Titration Chemical Reaction learn about the four types of titration: titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn about the four types of titration:
From www.freepik.com
Premium AI Image Colorful chemical reaction of titration in a laboratory Titration Chemical Reaction a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn about the four types of titration: titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. titration is a method of quantitative chemical analysis to determine. Titration Chemical Reaction.
From studylib.net
Diprotic Titration MultiStep Chemical Reactions Titration Chemical Reaction learn about the four types of titration: titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. titration is a technique to determine the. Titration Chemical Reaction.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Chemical Reaction learn about the four types of titration: Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. a titration is a laboratory technique to measure the. Titration Chemical Reaction.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Chemical Reaction a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction.. Titration Chemical Reaction.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is a technique to determine the concentration of an. Titration Chemical Reaction.
From gioldvmyt.blob.core.windows.net
Titration Chemical Method at Karl Twiggs blog Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn about the four types of titration: learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. Find out the. Titration Chemical Reaction.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. a titration is a laboratory technique to measure the molar concentration of an. Titration Chemical Reaction.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Chemical Reaction titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn about the four types of titration: Find out the definitions, reactions, indicators. Titration Chemical Reaction.
From www.alamy.com
Chemical reaction titration experiment turns liquid from purple to Titration Chemical Reaction learn about the four types of titration: learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. a titration. Titration Chemical Reaction.
From www.dreamstime.com
Chemical Titration. Acidbase Balance Stock Photo Image of occupation Titration Chemical Reaction learn about the four types of titration: titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is. Titration Chemical Reaction.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Chemical Reaction titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. learn about the four types of titration: Find out the definitions, reactions, indicators and applications of. Titration Chemical Reaction.
From exymjkekq.blob.core.windows.net
Titration In Chemistry Equipment at Barbara Adamson blog Titration Chemical Reaction Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn about the four types of titration: titration is a method of quantitative chemical analysis to. Titration Chemical Reaction.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. Find out the definitions, reactions, indicators and applications of each type of titration with examples and. Titration Chemical Reaction.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titration Chemical Reaction titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. learn about the four types of titration: Find out the definitions, reactions, indicators and applications of. Titration Chemical Reaction.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Chemical Reaction titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a. Titration Chemical Reaction.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. learn how to measure the concentration of a substance in a solution using titration, a technique that. Titration Chemical Reaction.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn how to measure the concentration of a substance in a solution using titration,. Titration Chemical Reaction.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Chemical Reaction a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. learn how to measure the concentration of a substance in a solution using titration, a technique that involves. Titration Chemical Reaction.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. learn how to measure the concentration of a substance in a solution using titration, a. Titration Chemical Reaction.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Chemical Reaction Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. learn about the four types of titration: titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. a titration is a laboratory technique to measure the molar concentration of an unknown solution. Titration Chemical Reaction.
From www.youtube.com
REDOX REACTIONS AS THE BASIS FOR TITRATIONS YouTube Titration Chemical Reaction titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. learn about the four types of titration: a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. titration is a method of quantitative chemical analysis to determine. Titration Chemical Reaction.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Chemical Reaction titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. learn how. Titration Chemical Reaction.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Chemical Reaction Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of. Titration Chemical Reaction.
From www.showme.com
Titration Curves Calculations Science, Chemicalreactions, Chemistry Titration Chemical Reaction learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a technique to determine the concentration of an. Titration Chemical Reaction.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Chemical Reaction titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn how to measure the concentration of a substance in a solution using titration, a. Titration Chemical Reaction.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. titration is a technique to determine the concentration of an unknown solution by adding a. Titration Chemical Reaction.
From mungfali.com
Acid Base Titration Method Titration Chemical Reaction titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn about the four types of titration: Find out the definitions, reactions, indicators and applications of each type. Titration Chemical Reaction.
From byjus.com
Why is titration used? Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. titration is a technique to determine the concentration of an unknown solution by adding a known solution. Titration Chemical Reaction.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Chemical Reaction Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction. learn about the four types of titration: titration is a method of determining the quantity of a substance in a. Titration Chemical Reaction.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Chemical Reaction a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn about the four types of titration: titration is a technique to determine. Titration Chemical Reaction.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is a technique to determine the concentration of an. Titration Chemical Reaction.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. titration is a method of quantitative chemical analysis to determine the concentration of an. Titration Chemical Reaction.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog Titration Chemical Reaction a titration is a laboratory technique to measure the molar concentration of an unknown solution using a standard solution. learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. titration is a method of quantitative chemical analysis to determine the concentration of an analyte. Titration Chemical Reaction.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration Chemical Reaction learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known. Find out the definitions, reactions, indicators and applications of each type of titration with examples and videos. learn about the four types of titration: titration is a technique to determine the concentration of an. Titration Chemical Reaction.
From bramblechemistry.weebly.com
4C6 Titration Titration Chemical Reaction titration is a method of determining the quantity of a substance in a sample by adding a known amount of another substance that reacts. titration is a method of quantitative chemical analysis to determine the concentration of an analyte by reacting it. Find out the definitions, reactions, indicators and applications of each type of titration with examples and. Titration Chemical Reaction.