Dilution In Chemistry Meaning . a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Concentration is the removal of solvent, which. You can add water to. dilution is the addition of water to reduce concentration; Dilution does not change the number of moles; a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution changes the e value of the atoms or. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent.
from www.studocu.com
Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of water to reduce concentration; Dilution does not change the number of moles; Dilution changes the e value of the atoms or. You can add water to. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Concentration is the removal of solvent, which. learn how to dilute and concentrate solutions.
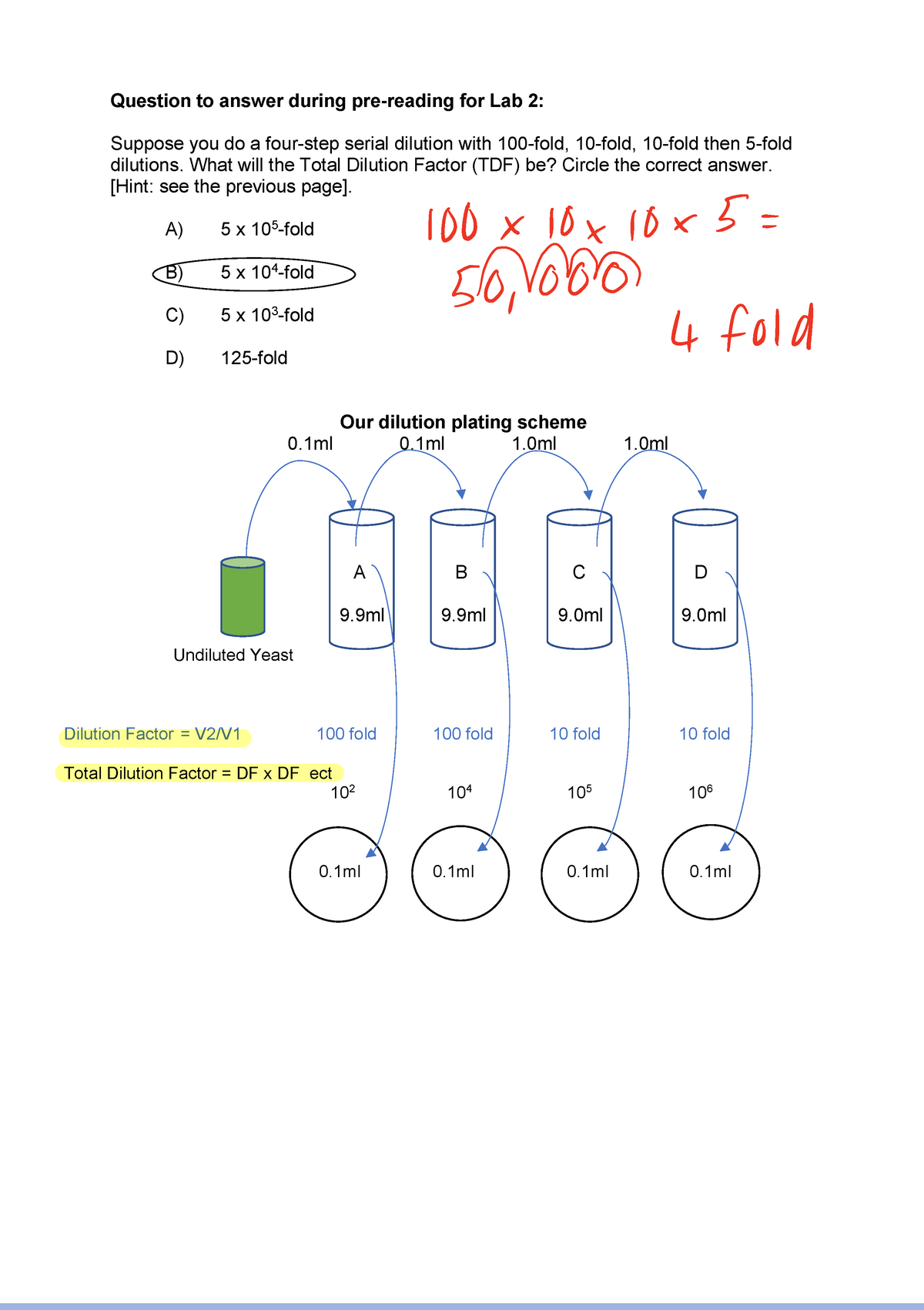
1. Serial Dilution Calculations Dilution Plating Questions Question
Dilution In Chemistry Meaning a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Concentration is the removal of solvent, which. learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of water to reduce concentration; Dilution changes the e value of the atoms or. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). You can add water to. Dilution does not change the number of moles; Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution In Chemistry Meaning a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). learn how to dilute and concentrate solutions. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Often, a worker will need to change the. Dilution In Chemistry Meaning.
From exoxgkatn.blob.core.windows.net
Dilute Solution Acid at Michael Keller blog Dilution In Chemistry Meaning You can add water to. learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which. Dilution does not change the number of moles; Dilution changes the e value of the atoms or. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of. Dilution In Chemistry Meaning.
From dxoybldvl.blob.core.windows.net
Dilution Meaning In Gujarati at Ella Albrecht blog Dilution In Chemistry Meaning a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of water to reduce concentration; Dilution does not change the number of. Dilution In Chemistry Meaning.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution In Chemistry Meaning dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Concentration is. Dilution In Chemistry Meaning.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions Dilution In Chemistry Meaning dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilution does not change the number of moles; Often, a worker will need to change the. Dilution In Chemistry Meaning.
From gioczvawq.blob.core.windows.net
Dilution Charts Chemicals at Robert Shaver blog Dilution In Chemistry Meaning Concentration is the removal of solvent, which. dilution is the addition of water to reduce concentration; dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution changes. Dilution In Chemistry Meaning.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution In Chemistry Meaning dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of water to reduce concentration; Dilution does not change the number of moles; a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. . Dilution In Chemistry Meaning.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution In Chemistry Meaning You can add water to. dilution is the addition of water to reduce concentration; Dilution does not change the number of moles; a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the addition of solvent, which decreases the concentration of the solute. Dilution In Chemistry Meaning.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution In Chemistry Meaning You can add water to. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. Dilution In Chemistry Meaning.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution In Chemistry Meaning Dilution changes the e value of the atoms or. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is the addition of solvent, which decreases the. Dilution In Chemistry Meaning.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution In Chemistry Meaning dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution changes the e value of the atoms or. You can add water to. dilution is the addition of water to reduce concentration; learn how to dilute and concentrate solutions. a dilute solution is one in. Dilution In Chemistry Meaning.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution In Chemistry Meaning a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of water to reduce concentration; a solution containing a precise mass of solute. Dilution In Chemistry Meaning.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution In Chemistry Meaning dilution is the addition of water to reduce concentration; Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution does not change the number. Dilution In Chemistry Meaning.
From hereditybio.in
Serial Dilution Technique for Bacterial Quantification Procedure and Dilution In Chemistry Meaning learn how to dilute and concentrate solutions. dilution is the addition of water to reduce concentration; Dilution does not change the number of moles; Concentration is the removal of solvent, which. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of. Dilution In Chemistry Meaning.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution In Chemistry Meaning You can add water to. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Concentration is the removal of solvent, which. Dilution does not change the number of moles; dilution. Dilution In Chemistry Meaning.
From dalconhygiene.com.au
Chemical Dilution Rate Guide Dalcon Hygiene Dilution In Chemistry Meaning Dilution does not change the number of moles; dilution is the addition of water to reduce concentration; You can add water to. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which there is a relatively small amount. Dilution In Chemistry Meaning.
From www.slideshare.net
Serial dilution Dilution In Chemistry Meaning Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to. Dilution In Chemistry Meaning.
From socratic.org
How many milliliters of 12.0 M HCl(aq) must be diluted with water to Dilution In Chemistry Meaning a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Concentration is the removal of solvent, which. dilution is the process of reducing the concentration of a solute. Dilution In Chemistry Meaning.
From pressurewashingresource.com
Chem dilution chart Chemical/Chemistry Pressure Washing Resource Dilution In Chemistry Meaning Dilution changes the e value of the atoms or. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to dilute and concentrate solutions. Dilution does not change the number of moles; Concentration is the removal of solvent, which. Often, a worker will need to change the. Dilution In Chemistry Meaning.
From carlosgokeowen.blogspot.com
What is Dilution Dilution In Chemistry Meaning Concentration is the removal of solvent, which. dilution is the addition of water to reduce concentration; a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to. Dilution In Chemistry Meaning.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution In Chemistry Meaning You can add water to. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. a solution containing a precise mass of solute in a precise volume of. Dilution In Chemistry Meaning.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution In Chemistry Meaning dilution is the addition of water to reduce concentration; Concentration is the removal of solvent, which. You can add water to. Dilution changes the e value of the atoms or. learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the. Dilution In Chemistry Meaning.
From studyadvertiser.z21.web.core.windows.net
Calculate Final Concentration After Dilution Dilution In Chemistry Meaning Dilution does not change the number of moles; Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). dilution is. Dilution In Chemistry Meaning.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Dilution In Chemistry Meaning dilution is the addition of water to reduce concentration; learn how to dilute and concentrate solutions. Dilution changes the e value of the atoms or. Dilution does not change the number of moles; dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. You. Dilution In Chemistry Meaning.
From dxowewdcl.blob.core.windows.net
Dilution Chemical Meaning at Sylvia Waldon blog Dilution In Chemistry Meaning a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). learn how to dilute and concentrate solutions. Dilution changes the e value of the atoms or. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the. Dilution In Chemistry Meaning.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution In Chemistry Meaning dilution is the addition of water to reduce concentration; Dilution does not change the number of moles; Dilution changes the e value of the atoms or. You can add water to. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of solvent,. Dilution In Chemistry Meaning.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution In Chemistry Meaning Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard. Dilution In Chemistry Meaning.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution In Chemistry Meaning dilution is the addition of water to reduce concentration; learn how to dilute and concentrate solutions. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often,. Dilution In Chemistry Meaning.
From blendwell.blogspot.com
Blendwell Chemicals How to Dilute Cleaning Chemicals Dilution In Chemistry Meaning learn how to dilute and concentrate solutions. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing. Dilution In Chemistry Meaning.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Dilution In Chemistry Meaning dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. learn how to dilute and concentrate solutions. dilution is the addition of water to reduce concentration; Dilution changes the e value of the atoms or. Concentration is the removal of solvent, which. You can add water to.. Dilution In Chemistry Meaning.
From dxorsyqnq.blob.core.windows.net
Dilution Adjective Definition at Robert Gonzales blog Dilution In Chemistry Meaning Concentration is the removal of solvent, which. Dilution changes the e value of the atoms or. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration. Dilution In Chemistry Meaning.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution In Chemistry Meaning a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution does not change the number of moles; Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the addition of solvent, which decreases the concentration of the solute. Dilution In Chemistry Meaning.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution In Chemistry Meaning You can add water to. dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilution does not change the number of moles; learn how. Dilution In Chemistry Meaning.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilution In Chemistry Meaning Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. You can add water to. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Concentration is the removal of solvent,. Dilution In Chemistry Meaning.
From dxorvxlft.blob.core.windows.net
Serial Dilutions Lab at Joseph Brown blog Dilution In Chemistry Meaning Dilution changes the e value of the atoms or. dilution is the addition of water to reduce concentration; a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which. dilution is. Dilution In Chemistry Meaning.