Organic Solvent Used In Partition Coefficient . a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. In most cases, the organic solvent is not. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the.
from www.numerade.com
the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. In most cases, the organic solvent is not.
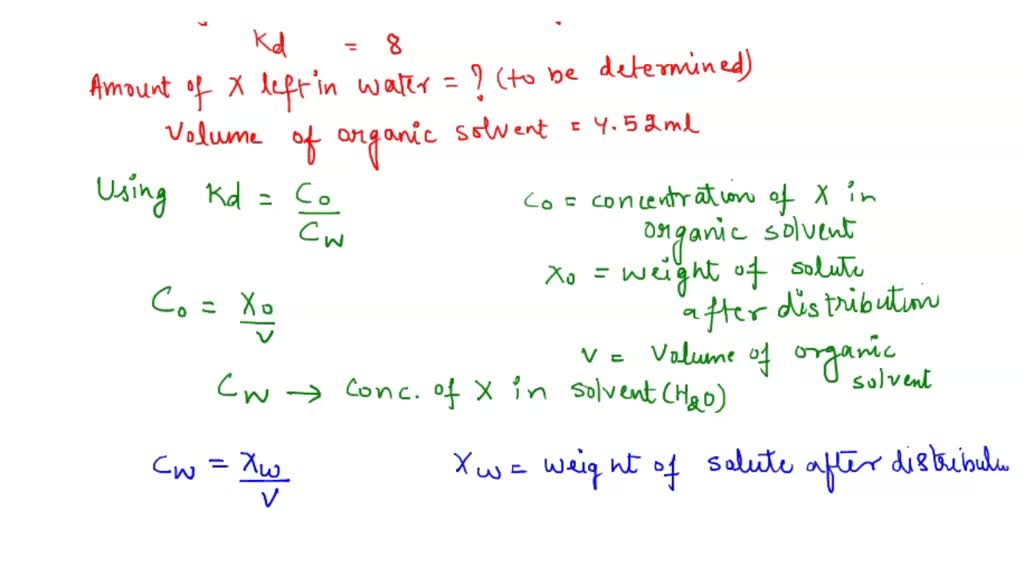
SOLVED The partition coefficient of Compound X is 11. Using the
Organic Solvent Used In Partition Coefficient revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. In most cases, the organic solvent is not. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams.
From www.chegg.com
Estimate the organic carbon partition coefficient Organic Solvent Used In Partition Coefficient revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. the partition coefficient is a measure of the distribution of a solute between two. Organic Solvent Used In Partition Coefficient.
From www.youtube.com
Kow part 1 partitioning between organic solvents and water YouTube Organic Solvent Used In Partition Coefficient the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. In most cases, the organic solvent is not. a solvent partitioning almost always involves the use of water. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
Solubilities of Leucine in Water and Organic Solvents, as log C, and Organic Solvent Used In Partition Coefficient in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon &. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
Chemical structures of the solvents. Download Scientific Diagram Organic Solvent Used In Partition Coefficient a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. In most cases, the organic solvent is not. partition coefficients are the ratio of the concentration. Organic Solvent Used In Partition Coefficient.
From www.youtube.com
Partition Coefficient Organic Chemistry Lab lecture YouTube Organic Solvent Used In Partition Coefficient partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. to overcome this issue, k d is often normalized to the organic carbon content. Organic Solvent Used In Partition Coefficient.
From www.numerade.com
SOLVED 3. A weak acid A with Ka = 1.82 x 105 has a partition Organic Solvent Used In Partition Coefficient a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. partition coefficients are the ratio of the concentration of an organic compound in two phases that. Organic Solvent Used In Partition Coefficient.
From dokumen.tips
(PDF) How does the polarity of organic solvents affect to the · The Organic Solvent Used In Partition Coefficient partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents. Organic Solvent Used In Partition Coefficient.
From www.youtube.com
Organic Lab Partition Coefficient 001 YouTube Organic Solvent Used In Partition Coefficient a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. In most cases, the organic solvent is not. revision notes on 5.5.9 partition coefficients for the. Organic Solvent Used In Partition Coefficient.
From digital.library.unt.edu
Prediction of Partition Coeffecients and Permeability of Drug Molecules Organic Solvent Used In Partition Coefficient the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in a. Organic Solvent Used In Partition Coefficient.
From www.scribd.com
Determination of Distribution Coefficient of Iodine Between Two Organic Solvent Used In Partition Coefficient in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. In most cases, the organic solvent is not. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. a solvent partitioning almost always involves the use of. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
(PDF) Study of thrmodynamic values in different temperature from Organic Solvent Used In Partition Coefficient the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. In most cases, the organic solvent is not. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. in a multiple extraction procedure, a quantity of solvent is used to extract one. Organic Solvent Used In Partition Coefficient.
From www.youtube.com
Solvent Extraction with Example Chapter 2F.Sc Chemistry Part1 Organic Solvent Used In Partition Coefficient the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. In most cases, the organic solvent is not. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. partition coefficients are the ratio of the concentration of an organic compound in two. Organic Solvent Used In Partition Coefficient.
From docslib.org
And OctanolWater Partition Coefficient (Kow) Data for Hydrophobic Organic Solvent Used In Partition Coefficient In most cases, the organic solvent is not. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. a solvent partitioning almost always involves the use of water. Organic Solvent Used In Partition Coefficient.
From www.myxxgirl.com
Partition Coefficient For Organic Acids My XXX Hot Girl Organic Solvent Used In Partition Coefficient to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save. Organic Solvent Used In Partition Coefficient.
From chem.libretexts.org
4.5 Extraction Theory Chemistry LibreTexts Organic Solvent Used In Partition Coefficient the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with. Organic Solvent Used In Partition Coefficient.
From thechemistrynotes.com
Partition coefficient (P)/ Distribution coefficient (D) Organic Solvent Used In Partition Coefficient to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium. Organic Solvent Used In Partition Coefficient.
From www.studocu.com
Organic chemistry Lab report number 1 Solvent Extraction and Organic Solvent Used In Partition Coefficient revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to. Organic Solvent Used In Partition Coefficient.
From www.scribd.com
Partition Coefficient Edited Solvent Solubility Organic Solvent Used In Partition Coefficient in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. partition coefficients are the ratio of the concentration of an organic compound in two phases. Organic Solvent Used In Partition Coefficient.
From www.slideserve.com
PPT Equilibrium Chemistry PowerPoint Presentation, free download ID Organic Solvent Used In Partition Coefficient partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. In most cases, the organic solvent is not. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. to overcome this issue, k d is often normalized to. Organic Solvent Used In Partition Coefficient.
From dokumen.tips
(PDF) Universal Organic SolventWater Partition Coefficient Model Organic Solvent Used In Partition Coefficient in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon. Organic Solvent Used In Partition Coefficient.
From www.numerade.com
SOLVED The partitioning coefficient (P) describes the distribution of Organic Solvent Used In Partition Coefficient partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in a multiple extraction procedure, a quantity of solvent is used to extract one. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
(PDF) Effect of solutes structure and pH on the noctanol/water Organic Solvent Used In Partition Coefficient a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). In most cases, the organic solvent is not. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. the octanol/water and octanol/air partition coefficients are predicted for a. Organic Solvent Used In Partition Coefficient.
From www.youtube.com
Sorption 4 Koc, the organic carbonwater partition coefficient YouTube Organic Solvent Used In Partition Coefficient the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. In most cases,. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
(PDF) Relation between partition coefficient of a weak organic base and Organic Solvent Used In Partition Coefficient revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. In most cases, the organic solvent is not. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). the partition coefficient is a measure of the. Organic Solvent Used In Partition Coefficient.
From www.numerade.com
SOLVEDPARTITION EQUILIBRIA and SOLVENT EXTRACTION The partition Organic Solvent Used In Partition Coefficient revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. a solvent partitioning almost always involves the use of water and an organic. Organic Solvent Used In Partition Coefficient.
From employees.csbsju.edu
Solvent Partitioning Organic Solvent Used In Partition Coefficient the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. . Organic Solvent Used In Partition Coefficient.
From studylib.net
Partition coefficient and partition calculations Organic Solvent Used In Partition Coefficient a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. to overcome this issue, k d is often normalized to the organic carbon content of the. Organic Solvent Used In Partition Coefficient.
From www.numerade.com
SOLVED The partitioning coefficient (P) describes the distribution of Organic Solvent Used In Partition Coefficient the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. to overcome this issue, k d is often normalized to the organic carbon content of the matrix. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
Butanol and water partition and solubility data in various organic Organic Solvent Used In Partition Coefficient the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. partition coefficients are the ratio of the concentration of an organic compound in two phases that are. Organic Solvent Used In Partition Coefficient.
From www.researchgate.net
Correlation between the octanolwater partition coefficient of several... Organic Solvent Used In Partition Coefficient revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. In most cases, the organic solvent is not. the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. partition coefficients are the ratio of the concentration of. Organic Solvent Used In Partition Coefficient.
From www.numerade.com
SOLVED The partition coefficient of Compound X is 11. Using the Organic Solvent Used In Partition Coefficient to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by the chemistry experts at save. Organic Solvent Used In Partition Coefficient.
From www.templateroller.com
Common Organic Solvents Table of Properties Yellow Download Printable Organic Solvent Used In Partition Coefficient the octanol/water and octanol/air partition coefficients are predicted for a group of polar solvents using density functional. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). In most cases, the organic solvent is not. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus,. Organic Solvent Used In Partition Coefficient.
From www.youtube.com
Solvent Extraction Partition Coefficient Questions YouTube Organic Solvent Used In Partition Coefficient in a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in. to overcome this issue, k d is often normalized to the organic carbon content of the matrix to determine the. revision notes on 5.5.9 partition coefficients for the cie a level chemistry syllabus, written by. Organic Solvent Used In Partition Coefficient.
From www.numerade.com
SOLVED A 52.0gram sample of an organic solid is dissolved in 110mL of Organic Solvent Used In Partition Coefficient the partition coefficient is a measure of the distribution of a solute between two immiscible phases, typically a. partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. In most cases, the organic solvent is not. in a multiple extraction procedure, a quantity of solvent is. Organic Solvent Used In Partition Coefficient.
From dokumen.tips
(PDF) Calculating the Partition Coefficients of Organic Solvents Organic Solvent Used In Partition Coefficient a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). partition coefficients are the ratio of the concentration of an organic compound in two phases that are in equilibrium with each. In most cases, the organic solvent is not. to overcome this issue, k d is often normalized. Organic Solvent Used In Partition Coefficient.