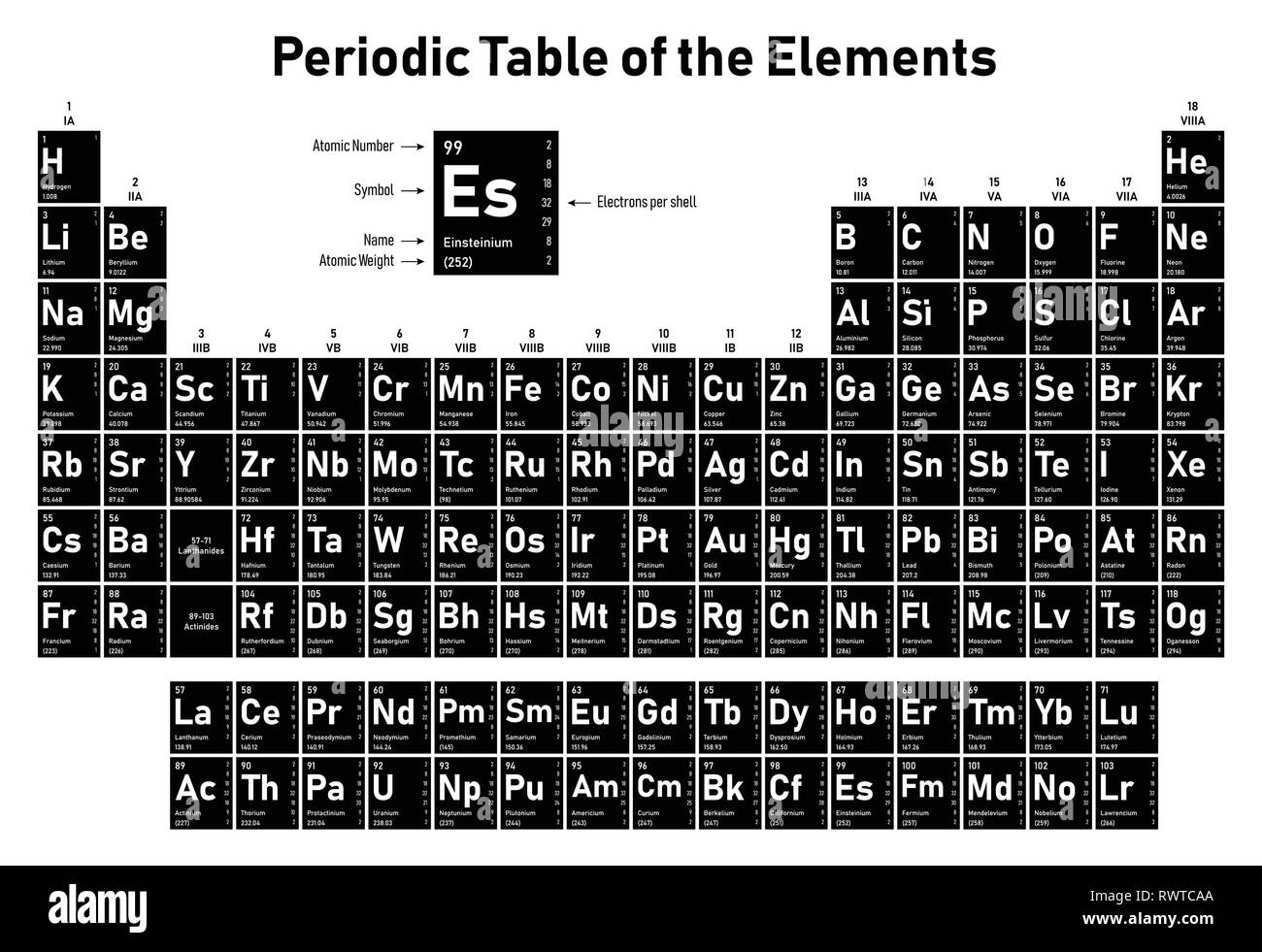
Iron Electrons Per Shell . how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. iron is a chemical element of the periodic table with chemical symbol fe and atomic. — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. The electron filling depends upon the energy levels of the. As a result, iron has eight valence electrons. However, it's easy to determine the configuration of electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The third shell can carry up 18 electrons,.
from www.alamy.com
— elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. The third shell can carry up 18 electrons,. However, it's easy to determine the configuration of electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. As a result, iron has eight valence electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. The electron filling depends upon the energy levels of the.
Periodic Table of the Elements shows atomic number, symbol, name
Iron Electrons Per Shell — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. As a result, iron has eight valence electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. The third shell can carry up 18 electrons,. The electron filling depends upon the energy levels of the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the.
From www.istockphoto.com
Fe Iron Element Information Facts Properties Trends Uses And Comparison Iron Electrons Per Shell As a result, iron has eight valence electrons. However, it's easy to determine the configuration of electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. how to write the electron configuration for iron. Iron Electrons Per Shell.
From www.alamy.com
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron Iron Electrons Per Shell As a result, iron has eight valence electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. The third shell can carry up 18 electrons,. The electron filling depends upon the energy levels of the. However, it's easy to determine the configuration of electrons. — its not necessary that 3rd shell has. Iron Electrons Per Shell.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Electrons Per Shell However, it's easy to determine the configuration of electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The electron filling depends upon the energy levels of the. — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. how to write the electron configuration. Iron Electrons Per Shell.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo (Free Trial) Bigstock Iron Electrons Per Shell — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. As a result, iron has eight. Iron Electrons Per Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Iron Electrons Per Shell However, it's easy to determine the configuration of electrons. The electron filling depends upon the energy levels of the. iron is a chemical element of the periodic table with chemical symbol fe and atomic. As a result, iron has eight valence electrons. The third shell can carry up 18 electrons,. — its not necessary that 3rd shell has. Iron Electrons Per Shell.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electrons Per Shell — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). As a result, iron has eight valence electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. how. Iron Electrons Per Shell.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name Iron Electrons Per Shell — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. The electron filling depends upon the energy levels of the. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd shell has to have 18 electrons for 4th shell. Iron Electrons Per Shell.
From en-academic.com
Iron Iron Electrons Per Shell iron is a chemical element of the periodic table with chemical symbol fe and atomic. As a result, iron has eight valence electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. —. Iron Electrons Per Shell.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413991 Iron Electrons Per Shell — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. The electron filling depends upon the energy levels of the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. — elements are shown from. Iron Electrons Per Shell.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Electrons Per Shell The third shell can carry up 18 electrons,. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. As a result, iron has eight valence electrons. However, it's easy to determine the configuration of electrons. The electron filling depends upon the energy levels of the. . Iron Electrons Per Shell.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electrons Per Shell As a result, iron has eight valence electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. — its not necessary that 3rd shell has to have 18 electrons for 4th shell. Iron Electrons Per Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica Iron Electrons Per Shell The third shell can carry up 18 electrons,. iron is a chemical element of the periodic table with chemical symbol fe and atomic. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. As a. Iron Electrons Per Shell.
From mungfali.com
How Many Electrons Are In Each Shell Iron Electrons Per Shell iron is a chemical element of the periodic table with chemical symbol fe and atomic. As a result, iron has eight valence electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. However, it's easy to determine the configuration of electrons. how to write the electron configuration. Iron Electrons Per Shell.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Electrons Per Shell — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. As a result, iron has eight valence electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons. The electron filling depends upon the energy levels of. Iron Electrons Per Shell.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electrons Per Shell However, it's easy to determine the configuration of electrons. — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The third shell can carry up 18 electrons,. how to write the electron configuration for iron. Iron Electrons Per Shell.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electrons Per Shell The electron filling depends upon the energy levels of the. However, it's easy to determine the configuration of electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd. Iron Electrons Per Shell.
From whatsinsight.org
How many valence electrons does iron have? What's Insight Iron Electrons Per Shell — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. However, it's easy to determine the configuration of electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. The electron filling depends upon the energy levels of the. The third shell can carry. Iron Electrons Per Shell.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Iron Electrons Per Shell — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). iron is a chemical element of the periodic table with chemical symbol fe and atomic. As a result, iron has eight valence electrons. how. Iron Electrons Per Shell.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electrons Per Shell However, it's easy to determine the configuration of electrons. The third shell can carry up 18 electrons,. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. As a result, iron has eight valence. Iron Electrons Per Shell.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png Iron Electrons Per Shell — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. —. Iron Electrons Per Shell.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electrons Per Shell how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. As a result, iron has eight valence electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. The electron filling depends upon the energy levels of the. —. Iron Electrons Per Shell.
From utedzz.blogspot.com
Periodic Table Electrons Per Shell Periodic Table Timeline Iron Electrons Per Shell The third shell can carry up 18 electrons,. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. However, it's easy to determine the configuration of. Iron Electrons Per Shell.
From www.scienceabc.com
Noble Metal Definition, List, Properties, And Examples Iron Electrons Per Shell The third shell can carry up 18 electrons,. The electron filling depends upon the energy levels of the. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. As a result, iron has eight valence electrons. — its not necessary that 3rd shell has to have 18 electrons for. Iron Electrons Per Shell.
From sciencenotes.org
Periodic Table Showing Shells Iron Electrons Per Shell The electron filling depends upon the energy levels of the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). iron is a chemical element of the periodic table with chemical. Iron Electrons Per Shell.
From dxoiremot.blob.core.windows.net
Element Iron Works at Robert Davis blog Iron Electrons Per Shell — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The third shell can carry up 18 electrons,. As a result, iron has eight valence electrons. The electron filling depends upon the energy levels of the. However, it's easy to determine the configuration of electrons. how to write the electron configuration for iron (fe) in. Iron Electrons Per Shell.
From www.slideserve.com
PPT Atoms the building blocks PowerPoint Presentation, free Iron Electrons Per Shell The third shell can carry up 18 electrons,. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons. The electron filling depends upon the energy levels of the. — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to. Iron Electrons Per Shell.
From www.alamy.com
Iron atom hires stock photography and images Alamy Iron Electrons Per Shell — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. As a result, iron has eight valence electrons. The electron filling depends upon the energy levels. Iron Electrons Per Shell.
From pediabay.com
Periodic Table & Energy Levels (Electrons per shell) Pediabay Iron Electrons Per Shell — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). iron is a chemical element of the periodic table with chemical symbol fe and atomic. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. — its not necessary that 3rd shell has to. Iron Electrons Per Shell.
From www.technocrazed.com
Principal quantum number n and maximum number of electrons per shell Iron Electrons Per Shell iron is a chemical element of the periodic table with chemical symbol fe and atomic. However, it's easy to determine the configuration of electrons. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. — its not necessary that 3rd shell has to have. Iron Electrons Per Shell.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Iron Electrons Per Shell The electron filling depends upon the energy levels of the. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons. As a result,. Iron Electrons Per Shell.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Iron Electrons Per Shell iron is a chemical element of the periodic table with chemical symbol fe and atomic. However, it's easy to determine the configuration of electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. The. Iron Electrons Per Shell.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Iron Electrons Per Shell As a result, iron has eight valence electrons. — the first shell can carry up to two electrons, the second shell can carry up to eight electrons. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The third shell can carry up 18 electrons,. However, it's easy to determine the configuration of electrons. The. Iron Electrons Per Shell.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 Iron Electrons Per Shell As a result, iron has eight valence electrons. iron is a chemical element of the periodic table with chemical symbol fe and atomic. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. However, it's easy to determine the configuration of electrons. — elements. Iron Electrons Per Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Iron Electrons Per Shell The electron filling depends upon the energy levels of the. — elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons. — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. — the first shell can carry. Iron Electrons Per Shell.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor Iron Electrons Per Shell — its not necessary that 3rd shell has to have 18 electrons for 4th shell filling up to start. iron is a chemical element of the periodic table with chemical symbol fe and atomic. The third shell can carry up 18 electrons,. — the first shell can carry up to two electrons, the second shell can carry. Iron Electrons Per Shell.