What Is The Function Of The Zinc And Hcl Used In This Procedure . filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. Which type of respiration uses nitrate as final acceptor? what is the function of the zinc and hcl used in this procedure? Zinc and hcl are reagents that abiotically catalyze the reduction of. the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. What is the function of the zinc and hcl used in this procedure? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the.
from lpi.oregonstate.edu
What is the function of the zinc and hcl used in this procedure? Zinc and hcl are reagents that abiotically catalyze the reduction of. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: what is the function of the zinc and hcl used in this procedure? the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. Which type of respiration uses nitrate as final acceptor? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the.
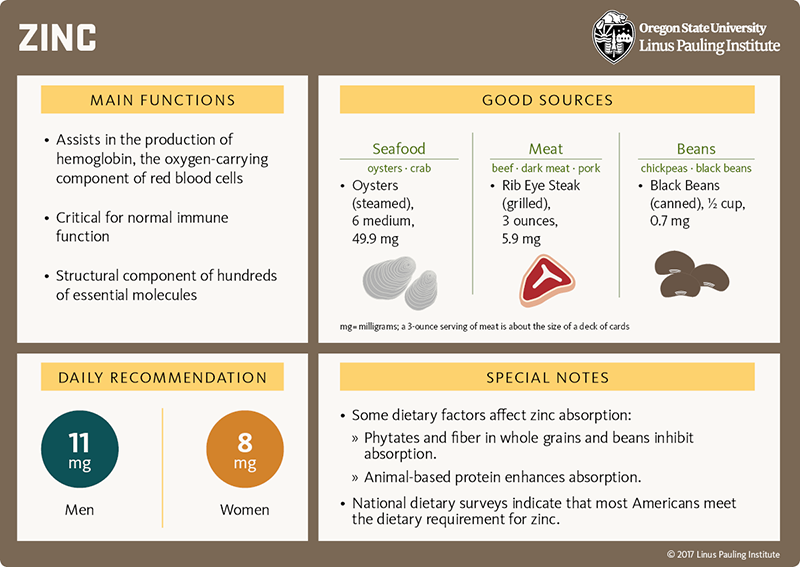
Immunity In Brief Linus Pauling Institute Oregon State University
What Is The Function Of The Zinc And Hcl Used In This Procedure a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. What is the function of the zinc and hcl used in this procedure? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. what is the function of the zinc and hcl used in this procedure? Which type of respiration uses nitrate as final acceptor? — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. Zinc and hcl are reagents that abiotically catalyze the reduction of. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction.
From www.researchgate.net
General description of zinc metal and zinc NPs concerning synthesis What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. What is the function of the zinc and hcl used in this procedure? when a zinc rod is inserted. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.semanticscholar.org
[PDF] The Physiological, Biochemical, and Molecular Roles of Zinc What Is The Function Of The Zinc And Hcl Used In This Procedure filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the What Is The Function Of The Zinc And Hcl Used In This Procedure a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. What is the function of the zinc and hcl used in this procedure? Which type of respiration uses nitrate as final acceptor? what is the function of the zinc and. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.glutenfreesociety.org
What Are The Functions of Zinc? Gluten Free Society What Is The Function Of The Zinc And Hcl Used In This Procedure filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. Zinc and hcl are reagents that abiotically catalyze the reduction of. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the.. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.chegg.com
Solved What is the function of the zinc and HCL used in What Is The Function Of The Zinc And Hcl Used In This Procedure — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. What is the function of the zinc and hcl used in this procedure? the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. what is the function of the zinc and. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.youtube.com
What are the Functions of Zinc YouTube What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. Which type of respiration uses nitrate. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From popasia.net
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration What Is The Function Of The Zinc And Hcl Used In This Procedure when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: what is the function of the zinc and hcl used in this procedure? What is the function of the zinc and hcl used in this procedure? the reaction between zinc metal and the hydrochloric acid. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.mdpi.com
Nutrients Free FullText Zinc as a Gatekeeper of Immune Function What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. Which type of respiration uses nitrate as final acceptor? What is the function of the zinc and hcl used in this procedure? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.glutenfreesociety.org
What Are The Functions of Zinc? Gluten Free Society What Is The Function Of The Zinc And Hcl Used In This Procedure the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. what is the function of the zinc and hcl used in this procedure? What is the function of the zinc. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From lpi.oregonstate.edu
Immunity In Brief Linus Pauling Institute Oregon State University What Is The Function Of The Zinc And Hcl Used In This Procedure What is the function of the zinc and hcl used in this procedure? Which type of respiration uses nitrate as final acceptor? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. when a zinc rod is inserted into a. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.glutenfreesociety.org
What Are The Functions of Zinc? Gluten Free Society What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. a typical cell might consist of two pieces of. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From express.adobe.com
Zinc and Hydrochloric Acid What Is The Function Of The Zinc And Hcl Used In This Procedure a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. What is the function of the zinc and hcl used in this procedure? — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.researchgate.net
The roles of zinc in human physiology. Some of the most important What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. a typical cell might consist of. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.elsevier.es
The role of zinc in liver cirrhosis Annals of Hepatology What Is The Function Of The Zinc And Hcl Used In This Procedure when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. — the zinc amalgam is used in. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.alamy.com
Labelled diagram for laboratory preparation of hydrogen from zinc and What Is The Function Of The Zinc And Hcl Used In This Procedure What is the function of the zinc and hcl used in this procedure? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. Zinc and hcl are reagents that abiotically catalyze the reduction of. the reaction between zinc metal and. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.glutenfreesociety.org
What Are The Functions of Zinc? GlutenFree Society What Is The Function Of The Zinc And Hcl Used In This Procedure — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. what is the function of the zinc and hcl used in this procedure? filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. What is the function of the zinc. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.youtube.com
Reaction of Zinc with HCl 10th class YouTube What Is The Function Of The Zinc And Hcl Used In This Procedure what is the function of the zinc and hcl used in this procedure? Zinc and hcl are reagents that abiotically catalyze the reduction of. the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. filtration and washing should be done as rapidly as possible to minimize exposure of the. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.numerade.com
SOLVED Consider the reaction of zinc metal with hydrochloric acid, HCl What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. What is the function of the zinc and hcl used in this procedure? the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From exonhfwss.blob.core.windows.net
How To Build Zinc In The Body at Siple blog What Is The Function Of The Zinc And Hcl Used In This Procedure what is the function of the zinc and hcl used in this procedure? — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. Which type of respiration uses nitrate as final acceptor? filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From oatext.com
An overview of zinc and its importance in dermatology Part I What Is The Function Of The Zinc And Hcl Used In This Procedure Which type of respiration uses nitrate as final acceptor? filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. What is the function of the zinc and hcl used in this procedure? — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From supremepharmatech.com
Liposomal Zinc supplement for Zinc deficiency What Is The Function Of The Zinc And Hcl Used In This Procedure what is the function of the zinc and hcl used in this procedure? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. What is the function of the zinc and hcl used in this procedure? Which type of respiration. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid What Is The Function Of The Zinc And Hcl Used In This Procedure when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: what is the function of the zinc and hcl used in this procedure? — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. Which type of respiration. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.youtube.com
Zinc and Hydrochloric Acid Reaction chemistry grade10science What Is The Function Of The Zinc And Hcl Used In This Procedure What is the function of the zinc and hcl used in this procedure? — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. a typical cell might consist of two. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid What Is The Function Of The Zinc And Hcl Used In This Procedure a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. what is the function of the zinc and hcl used in this procedure? Zinc and hcl are reagents that abiotically catalyze the reduction of. — the zinc amalgam is. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube What Is The Function Of The Zinc And Hcl Used In This Procedure What is the function of the zinc and hcl used in this procedure? Zinc and hcl are reagents that abiotically catalyze the reduction of. the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. filtration and washing should be done as rapidly as possible to minimize exposure of the activated. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From fitnessprogramer.com
What Is Zinc? Functions And Health Benefits What Is The Function Of The Zinc And Hcl Used In This Procedure a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. what is the function of the zinc and hcl used in. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science What Is The Function Of The Zinc And Hcl Used In This Procedure — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. the reaction between zinc metal and the hydrochloric acid solution is. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.researchgate.net
Zinc storage and distribution in the human body. The human body What Is The Function Of The Zinc And Hcl Used In This Procedure the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Which type of respiration uses nitrate as final acceptor? filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. when a zinc rod is inserted into a beaker that contains. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.youtube.com
ଜିଂକ ଉପରେ ଲଘୁ ହାଇଡ୍ରୋକ୍ଲୋରିକ ଏସିଡ୍ ର ପ୍ରତିକ୍ରିୟା / Zinc reacts with HCL What Is The Function Of The Zinc And Hcl Used In This Procedure when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. a typical cell might consist of two pieces of metal, one zinc and the other copper, each. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.glutenfreesociety.org
What Are The Functions of Zinc? Gluten Free Society What Is The Function Of The Zinc And Hcl Used In This Procedure when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: what is the function of the zinc and hcl used in this procedure? Zinc and hcl are reagents that abiotically catalyze the reduction of. a typical cell might consist of two pieces of metal, one. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.researchgate.net
Functions of zinc in human body Download Scientific Diagram What Is The Function Of The Zinc And Hcl Used In This Procedure filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Zinc and hcl are reagents that abiotically catalyze the reduction of. the reaction between zinc metal. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. What is the function of the zinc and hcl used in this procedure? Which type of respiration uses nitrate as final acceptor? a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From melscience.com
The reaction between hydrochloric acid and zinc MEL Chemistry What Is The Function Of The Zinc And Hcl Used In This Procedure the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Which type of respiration uses nitrate as final acceptor? when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: Zinc and hcl are reagents that abiotically catalyze the. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.researchgate.net
Functions of zinc in human body Download Scientific Diagram What Is The Function Of The Zinc And Hcl Used In This Procedure — the zinc amalgam is used in clemmensen reduction as the reaction of zinc with hydrochloric acid releases. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: a typical cell might consist of two pieces of metal, one zinc and the other copper, each. What Is The Function Of The Zinc And Hcl Used In This Procedure.
From www.mdpi.com
Biomolecules Free FullText The Important Role of Zinc in What Is The Function Of The Zinc And Hcl Used In This Procedure Zinc and hcl are reagents that abiotically catalyze the reduction of. when a zinc rod is inserted into a beaker that contains an aqueous solution of copper(ii) sulfate, a spontaneous redox reaction occurs: the reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. what is the function of the. What Is The Function Of The Zinc And Hcl Used In This Procedure.