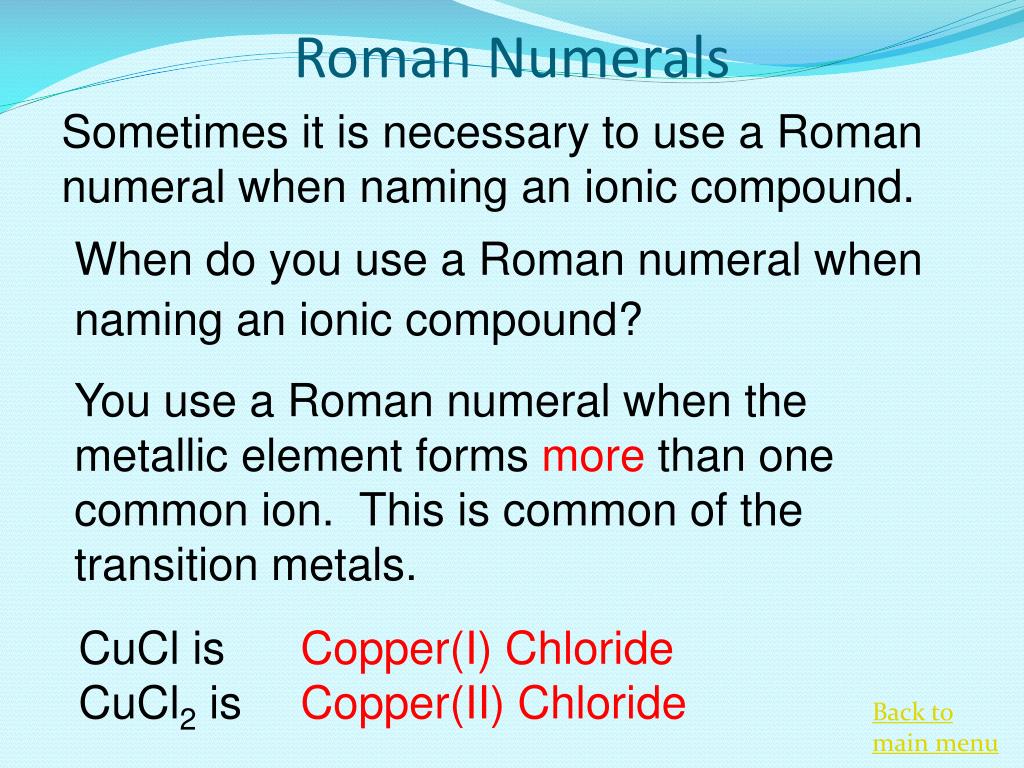
Copper Chloride Roman Numeral . So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. Substances with carbon and hydrogen are organic. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. nickel is a type ii cation, so you need a roman numeral. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. To figure out that charge x, we need take a look at what we know. ← chemical reactions · formulas and numbers →. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal.
from www.slideserve.com
roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. ← chemical reactions · formulas and numbers →. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. Substances with carbon and hydrogen are organic. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. nickel is a type ii cation, so you need a roman numeral. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. To figure out that charge x, we need take a look at what we know.
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free
Copper Chloride Roman Numeral So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. Substances with carbon and hydrogen are organic. ← chemical reactions · formulas and numbers →. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. To figure out that charge x, we need take a look at what we know. nickel is a type ii cation, so you need a roman numeral. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation.
From slideplayer.com
COMPOUNDS FORMED FROM IONS ppt download Copper Chloride Roman Numeral nickel is a type ii cation, so you need a roman numeral. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. Substances with carbon and hydrogen are organic. ← chemical reactions · formulas and numbers →. To figure out that charge x, we need take a look at what we know.. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Nomenclature Formula Writing & Naming Compounds ppt download Copper Chloride Roman Numeral roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. To figure out that charge x, we need take a look at what we know. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. ← chemical reactions. Copper Chloride Roman Numeral.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Copper Chloride Roman Numeral roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. nickel is a type ii cation, so you need a roman numeral. ← chemical reactions · formulas. Copper Chloride Roman Numeral.
From slideplayer.com
Three Fundamental Laws ppt download Copper Chloride Roman Numeral nickel is a type ii cation, so you need a roman numeral. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. To figure out that charge x, we need take a look at what we know. Substances with carbon and hydrogen are organic.. Copper Chloride Roman Numeral.
From slideplayer.com
(Putting Elements Together) ppt download Copper Chloride Roman Numeral metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. roman numerals are used in naming. Copper Chloride Roman Numeral.
From slideplayer.com
Nomenclature Naming Chemicals ppt download Copper Chloride Roman Numeral Substances with carbon and hydrogen are organic. nickel is a type ii cation, so you need a roman numeral. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. ← chemical reactions · formulas and numbers →. in such cases, the positive charge on the metal is indicated by a roman. Copper Chloride Roman Numeral.
From davida.davivienda.com
Free Printable Roman Numeral Chart Printable Word Searches Copper Chloride Roman Numeral metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. To figure out that charge x, we need take a look at what we know. nickel is a type ii cation, so you need a roman numeral. So, hg 2 cl 2 is mercury (ii) chloride. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Bonding Lesson 1 Ionic Bonds & Compounds. ppt download Copper Chloride Roman Numeral in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. ← chemical reactions · formulas and numbers →. nickel is a type ii cation, so you need a roman numeral. Substances with carbon and hydrogen are organic. So, hg 2 cl 2 is. Copper Chloride Roman Numeral.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Copper Chloride Roman Numeral metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of. Copper Chloride Roman Numeral.
From nicholasacademy.com
Kids Roman Numeral Chart 1 to 20 Printable Learn Roman Numbers Letters Copper Chloride Roman Numeral Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. To figure out that charge x, we need take a look at what we know. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. So, hg 2 cl. Copper Chloride Roman Numeral.
From www.numerade.com
SOLVEDThe compound CaCl2 is named calcium chloride; the compound CuCl2 Copper Chloride Roman Numeral Substances with carbon and hydrogen are organic. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. To figure out that charge x, we need take a look at what we know. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. metals in group 1 (alkali metals) and. Copper Chloride Roman Numeral.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Copper Chloride Roman Numeral metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. Substances with carbon and hydrogen are organic. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. ← chemical reactions · formulas and numbers →. To figure out. Copper Chloride Roman Numeral.
From slideplayer.com
Chapter 7 Language of Chemistry by Christopher Hamaker ppt download Copper Chloride Roman Numeral nickel is a type ii cation, so you need a roman numeral. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. To figure out that charge x, we need take a look at what we know. Unlike covalent compound names, there is no prefix indicating. Copper Chloride Roman Numeral.
From mavink.com
Roman Numerals Full Chart Copper Chloride Roman Numeral ← chemical reactions · formulas and numbers →. Substances with carbon and hydrogen are organic. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. nickel is. Copper Chloride Roman Numeral.
From romansnumerals.com
Printable Roman Numerals 1 to 20 Roman Numerals Pro Copper Chloride Roman Numeral metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. To figure out that charge x, we need take a look at what we know. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. in such cases, the positive charge on. Copper Chloride Roman Numeral.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Copper Chloride Roman Numeral Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. nickel is a type ii cation, so you need a roman numeral. ← chemical reactions · formulas and numbers →. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. in such cases,. Copper Chloride Roman Numeral.
From slideplayer.com
Names and Formulas of Ionic Compounds ppt download Copper Chloride Roman Numeral roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. Substances with carbon and hydrogen are organic. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. So, hg 2 cl 2 is mercury (ii) chloride and. Copper Chloride Roman Numeral.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download ID2170105 Copper Chloride Roman Numeral roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. To figure out that charge x, we need take a look at what we know. in such cases, the positive charge on the metal is. Copper Chloride Roman Numeral.
From utedzz.blogspot.com
Periodic Table Roman Numerals Periodic Table Timeline Copper Chloride Roman Numeral So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. Substances with carbon and hydrogen are organic. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of. Copper Chloride Roman Numeral.
From slideplayer.com
Atoms, Molecules, and Ions ppt download Copper Chloride Roman Numeral So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. nickel is a type ii cation, so you need a roman numeral. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. Substances with carbon and hydrogen are organic. metals in group 1 (alkali metals) and. Copper Chloride Roman Numeral.
From www.slideserve.com
PPT Chemical Nomenclature Naming compounds and writing chemical Copper Chloride Roman Numeral To figure out that charge x, we need take a look at what we know. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. nickel is a type ii cation, so you need a roman numeral. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. metals. Copper Chloride Roman Numeral.
From courses.lumenlearning.com
Lewis Dot Symbols and Lewis Structures Boundless Chemistry Copper Chloride Roman Numeral roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. nickel is a type ii cation, so you need a roman numeral. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. Substances with carbon and. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Nomenclature ppt download Copper Chloride Roman Numeral ← chemical reactions · formulas and numbers →. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. roman numerals are used in naming ionic compounds when the metal cation forms. Copper Chloride Roman Numeral.
From slideplayer.com
Bonding. ppt download Copper Chloride Roman Numeral nickel is a type ii cation, so you need a roman numeral. Substances with carbon and hydrogen are organic. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. roman numerals. Copper Chloride Roman Numeral.
From slideplayer.com
Compound Names & Formulas ppt download Copper Chloride Roman Numeral So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. Substances with carbon and hydrogen are organic. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. Unlike covalent compound names, there is no prefix indicating the number of atoms in. Copper Chloride Roman Numeral.
From www.youtube.com
Naming Ionic Compounds with Roman Numerals YouTube Copper Chloride Roman Numeral nickel is a type ii cation, so you need a roman numeral. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. To figure out that. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Bonds Chapter ppt download Copper Chloride Roman Numeral So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. Substances with carbon and hydrogen are organic. To figure out that charge x, we need take a look at what we know. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. . Copper Chloride Roman Numeral.
From www.youtube.com
Naming Compounds using Roman Numerals YouTube Copper Chloride Roman Numeral Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. ← chemical reactions · formulas and numbers →. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Reactions UNIT 2 Chapter 4 Developing Chemical Equations Copper Chloride Roman Numeral roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with no roman numerals. Substances with carbon and hydrogen are organic. nickel is a type ii cation, so you need a roman. Copper Chloride Roman Numeral.
From slideplayer.com
Ionic Compounds Naming and Writing Formulas ppt download Copper Chloride Roman Numeral in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. To figure out that charge x, we need take a look at what we know. metals in group 1 (alkali metals) and group 2 (alkaline earth metals) always go by their element name, with. Copper Chloride Roman Numeral.
From romansnumerals.com
Roman Numerals Converter & Chart 11000 in Roman Numerals Copper Chloride Roman Numeral ← chemical reactions · formulas and numbers →. Substances with carbon and hydrogen are organic. Unlike covalent compound names, there is no prefix indicating the number of atoms in the cation. To figure out that charge x, we need take a look at what we know. metals in group 1 (alkali metals) and group 2 (alkaline earth metals). Copper Chloride Roman Numeral.
From slideplayer.com
Unit 0 Nomenclature Naming Chemicals ppt download Copper Chloride Roman Numeral Substances with carbon and hydrogen are organic. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. roman numerals are used in naming ionic compounds when the metal cation forms. Copper Chloride Roman Numeral.
From slideplayer.com
Chapter 2 The Material World ppt download Copper Chloride Roman Numeral nickel is a type ii cation, so you need a roman numeral. To figure out that charge x, we need take a look at what we know. ← chemical reactions · formulas and numbers →. So, hg 2 cl 2 is mercury (ii) chloride and not dimercury (ii) dichloride. Substances with carbon and hydrogen are organic. in. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Nomenclature Formula Writing & Naming Compounds ppt download Copper Chloride Roman Numeral Substances with carbon and hydrogen are organic. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. metals in group 1 (alkali metals) and group 2. Copper Chloride Roman Numeral.
From slideplayer.com
Chemical Bonds. ppt download Copper Chloride Roman Numeral To figure out that charge x, we need take a look at what we know. in such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. ← chemical reactions · formulas and numbers →. Substances with carbon and hydrogen are organic. Unlike covalent compound names,. Copper Chloride Roman Numeral.