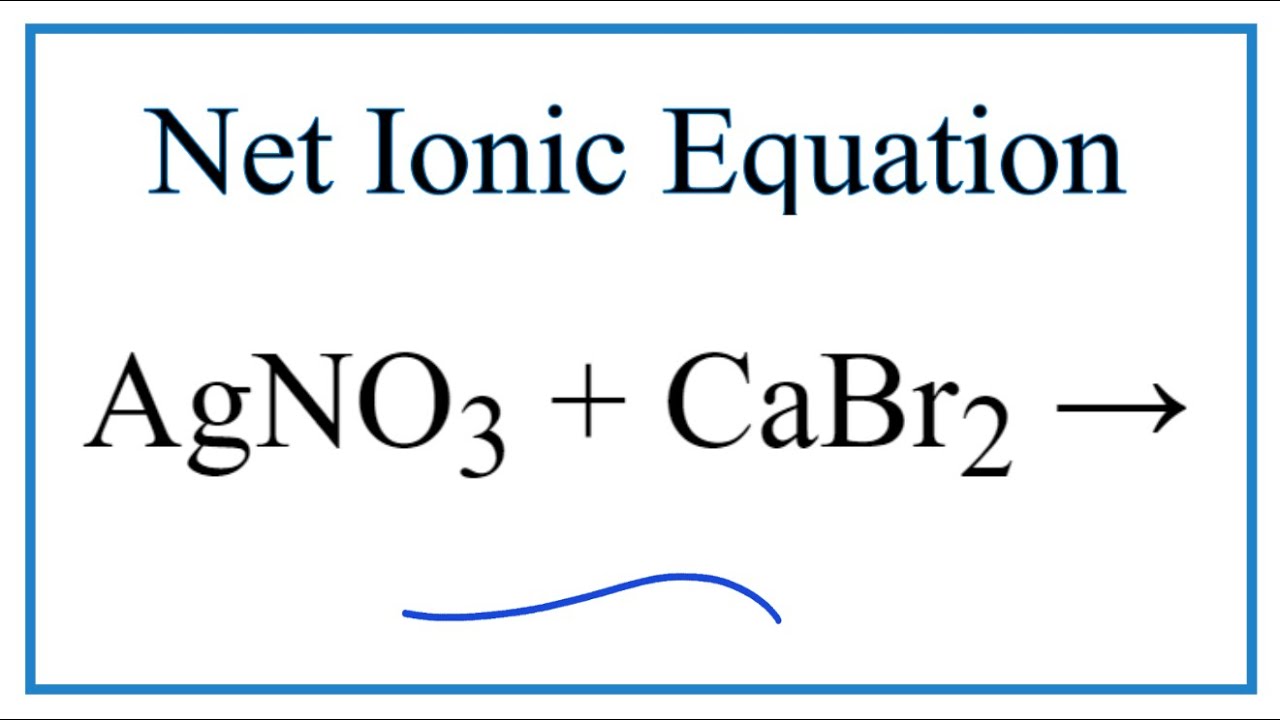Lead Carbonate + Hydrochloric Acid . This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. The reaction of a sodium carbonate solution. Occasionally, a reaction will produce both a gas and a molecular compound. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are.
from www.youtube.com
This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Occasionally, a reaction will produce both a gas and a molecular compound. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The reaction of a sodium carbonate solution. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess.
How to Write the Net Ionic Equation for AgNO3 + CaBr2 = Ca(NO3)2 + CaBr2 YouTube
Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reaction of a sodium carbonate solution. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Occasionally, a reaction will produce both a gas and a molecular compound.
From courses.lumenlearning.com
4.2 Classifying Chemical Reactions Chemistry Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water.. Lead Carbonate + Hydrochloric Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess.. Lead Carbonate + Hydrochloric Acid.
From www.slideserve.com
PPT Types of chemical reactions STUDY GUIDE V2.0 PowerPoint Presentation ID1758915 Lead Carbonate + Hydrochloric Acid Occasionally, a reaction will produce both a gas and a molecular compound. The reaction of a sodium carbonate solution. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. Lead Carbonate + Hydrochloric Acid.
From www.youtube.com
hydrochloric acid reacts with Sodium carbonate YouTube Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Occasionally, a reaction will produce both a gas and a molecular compound. The reason it reacts with concentrated. Lead Carbonate + Hydrochloric Acid.
From www.numerade.com
SOLVED '14. chromium (III) phosphide + iodide 15. potassium iodide + lead (II) acetate 16 Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Occasionally, a reaction will produce both a gas and a molecular compound. The reaction of a sodium carbonate solution. Write. Lead Carbonate + Hydrochloric Acid.
From www.labdepotinc.com
Lead Carbonate, Powder, Reagent, ACS Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess.. Lead Carbonate + Hydrochloric Acid.
From www.tessshebaylo.com
Balanced Chemical Equation Between Sodium Carbonate And Hydrochloric Acid Tessshebaylo Lead Carbonate + Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Occasionally, a. Lead Carbonate + Hydrochloric Acid.
From www.numerade.com
SOLVED Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution Do you Lead Carbonate + Hydrochloric Acid This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. The reaction of a sodium carbonate solution. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess.. Lead Carbonate + Hydrochloric Acid.
From www.thermofisher.com
Lead(II) carbonate, basic, extra pure, Thermo Scientific™ Lead Carbonate + Hydrochloric Acid Occasionally, a reaction will produce both a gas and a molecular compound. The reaction of a sodium carbonate solution. This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page looks at the formation. Lead Carbonate + Hydrochloric Acid.
From chemistrypubs.com
Standardization of Hydrochloric Acid with Standard Sodium Carbonate Solution Chemistrupubs Lead Carbonate + Hydrochloric Acid The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using. Lead Carbonate + Hydrochloric Acid.
From www.bartleby.com
Answered (d) ammonium carbonate and hydrochloric… bartleby Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Occasionally, a reaction will produce both a gas and a molecular compound. This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. The reason it reacts with concentrated hydrochloric acid is because of. Lead Carbonate + Hydrochloric Acid.
From summitchemmes.blogspot.com
Summit Chemistry Rox sodium carbonate and hydrochloric acid lab Lead Carbonate + Hydrochloric Acid Occasionally, a reaction will produce both a gas and a molecular compound. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Write a net ionic equation for. Lead Carbonate + Hydrochloric Acid.
From www.fishersci.ca
Lead(II) carbonate, ACS, Thermo Scientific Chemicals Fisher Scientific Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reaction of a sodium carbonate solution. Occasionally, a reaction will produce both a gas and a molecular compound. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide. Lead Carbonate + Hydrochloric Acid.
From www.youtube.com
COPPER(II) CARBONATE & HYDROCHLORIC ACID DEMONSTRATION YouTube Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reaction of a sodium carbonate solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The reason it reacts with concentrated hydrochloric acid is because of a further. Lead Carbonate + Hydrochloric Acid.
From www.grainger.com
598630, F.W. 267.20, Lead Carbonate, Powder, Reagent, ACS 39G576L1045125GM07 Grainger Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using. Lead Carbonate + Hydrochloric Acid.
From www.indiamart.com
Laboratory Lead Carbonate Liquid at Rs 120/bottle Lead Carbonate in Satara ID 21922164048 Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The reaction of a sodium carbonate solution. The reason it reacts with concentrated hydrochloric acid is because of a further. Lead Carbonate + Hydrochloric Acid.
From www.numerade.com
SOLVED Am asking on how a solid sample of lead ii chloride can be prepared using the following Lead Carbonate + Hydrochloric Acid The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Occasionally, a reaction will produce both a gas and a molecular compound. This page looks at the formation. Lead Carbonate + Hydrochloric Acid.
From www.slideshare.net
Reaction types Lead Carbonate + Hydrochloric Acid This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. The reaction of a sodium carbonate solution. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water. Lead Carbonate + Hydrochloric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for AgNO3 + CaBr2 = Ca(NO3)2 + CaBr2 YouTube Lead Carbonate + Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Occasionally, a reaction will produce both a gas and a molecular compound. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page discusses the precipitation of insoluble lead(ii). Lead Carbonate + Hydrochloric Acid.
From studymoose.com
Carbonate and hydrochloric acid Free Essay Example Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride. Lead Carbonate + Hydrochloric Acid.
From www.grainger.com
SPECTRUM Lead Carbonate, Powder, Reagent, ACS, 500g 39G579L1045500GM10 Grainger Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The reason. Lead Carbonate + Hydrochloric Acid.
From www.youtube.com
How to write the equation for PbCO3 + H2O Lead (II) carbonate + Water YouTube Lead Carbonate + Hydrochloric Acid Occasionally, a reaction will produce both a gas and a molecular compound. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. The reaction of a sodium carbonate solution. This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Write a net ionic equation for. Lead Carbonate + Hydrochloric Acid.
From www.adda247.com
Hydrochloric Acid Formula, HCL Density, Chemical Name, Uses Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions.. Lead Carbonate + Hydrochloric Acid.
From www.lennoxeducational.ie
13.2 To use a standard solution of sodium carbonate to standardise a given hydrochloric acid Lead Carbonate + Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. The reason. Lead Carbonate + Hydrochloric Acid.
From www.grainger.com
598630, F.W. 267.20, Lead Carbonate, Powder, Reagent, ACS 39G578L10452.5KG13 Grainger Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. Occasionally, a reaction will produce both a gas and a molecular compound. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. The reaction of a sodium carbonate solution. When acids react. Lead Carbonate + Hydrochloric Acid.
From www.youtube.com
How to Balance BaCO3 + HCl = BaCl2 + H2O + CO2 Barium carbonate + Hydrochloric acid YouTube Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. The reason. Lead Carbonate + Hydrochloric Acid.
From www.sciencephoto.com
Lead in hydrochloric acid Stock Image C043/6238 Science Photo Library Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions.. Lead Carbonate + Hydrochloric Acid.
From fyoawlxlr.blob.core.windows.net
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reaction of a sodium carbonate solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The reason it reacts with concentrated hydrochloric acid is because of a further. Lead Carbonate + Hydrochloric Acid.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Lead Carbonate + Hydrochloric Acid Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. Occasionally, a reaction will produce both a gas and a molecular compound. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. When acids react with carbonates, such as calcium carbonate (found. Lead Carbonate + Hydrochloric Acid.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation ID5408111 Lead Carbonate + Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. The reaction of a sodium carbonate solution. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water.. Lead Carbonate + Hydrochloric Acid.
From www.slideshare.net
Acids And Bases Lead Carbonate + Hydrochloric Acid Occasionally, a reaction will produce both a gas and a molecular compound. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the reactions. Lead Carbonate + Hydrochloric Acid.
From www.youtube.com
How to Write the Formula for Lead (II) carbonate YouTube Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. The reaction of a sodium carbonate solution. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page looks at the formation of some insoluble lead (ii). Lead Carbonate + Hydrochloric Acid.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP The Periodic Table Lead Carbonate + Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page discusses the precipitation of insoluble lead(ii) compounds from aqueous lead(ii) ions in solution. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. The reaction of. Lead Carbonate + Hydrochloric Acid.
From www.sciencephoto.com
Lead in hydrochloric acid Stock Image A500/0623 Science Photo Library Lead Carbonate + Hydrochloric Acid The reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and excess. Occasionally, a reaction will produce both a gas and a molecular compound. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Write a net ionic equation for the reaction that occurs. Lead Carbonate + Hydrochloric Acid.
From linativewise.blogspot.com
Calcium Carbonate and Hydrochloric Acid Lead Carbonate + Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Occasionally, a reaction will produce both a gas and a molecular compound. Write a net ionic equation for the reaction that occurs when hydrochloric acid (aq) and lead (ii) carbonate are combined. This page discusses the precipitation of insoluble lead(ii). Lead Carbonate + Hydrochloric Acid.