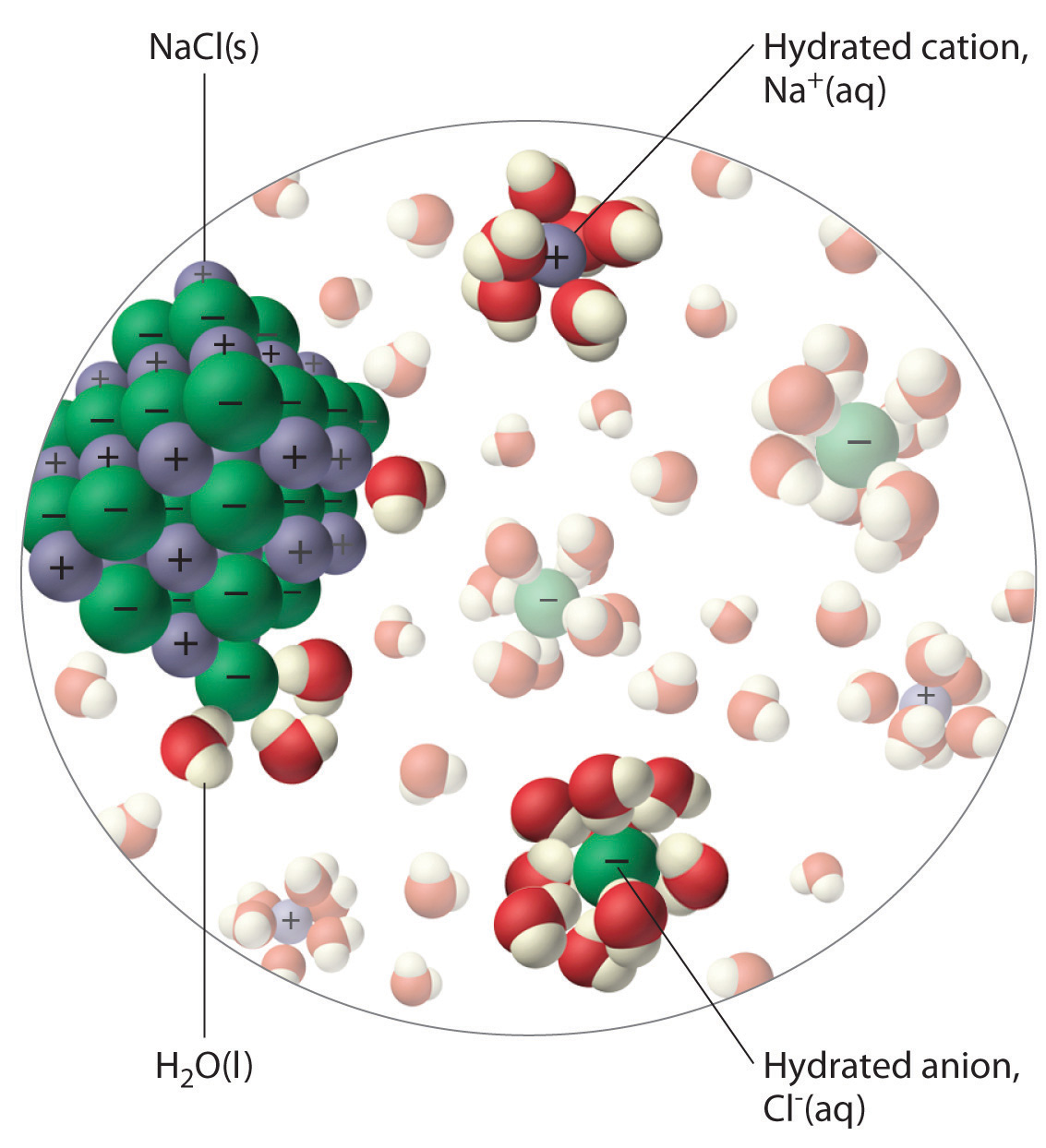
Chloride Ion And Water . We will first examine the process that occurs when. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. nonpolar molecules such as those found in grease or oil do not dissolve in water. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. water is attracted to the sodium chloride crystal because water is polar; this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. It has both a positive and a negative end.
from socratic.org
this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. nonpolar molecules such as those found in grease or oil do not dissolve in water. water is attracted to the sodium chloride crystal because water is polar; We will first examine the process that occurs when. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. It has both a positive and a negative end.
What is it called when sodium and chloride ions separate when dissolved
Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. It has both a positive and a negative end. nonpolar molecules such as those found in grease or oil do not dissolve in water. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. We will first examine the process that occurs when. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. water is attracted to the sodium chloride crystal because water is polar; the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation.
From depositphotos.com
Test Chloride Infographic Diagram Showing Laboratory Experiment Chloride Ion And Water Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. It has both a positive and a negative end. nonpolar molecules such as those found in grease or oil do not dissolve in water. the most common type of water in which chloride. Chloride Ion And Water.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Chloride Ion And Water We will first examine the process that occurs when. water is attracted to the sodium chloride crystal because water is polar; the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. Waters of this type tend to range from dilute solutions influenced by rainfall near the. Chloride Ion And Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Chloride Ion And Water We will first examine the process that occurs when. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. Waters of this. Chloride Ion And Water.
From stock.adobe.com
Vecteur Stock Ionic covalent bonds examples. Chemical structural models Chloride Ion And Water water is attracted to the sodium chloride crystal because water is polar; It has both a positive and a negative end. this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. the most common type of water in which chloride is the dominant anion. Chloride Ion And Water.
From www.researchgate.net
Electrostatic interaction of Na + and Cl¯ ions and water molecules Chloride Ion And Water water is attracted to the sodium chloride crystal because water is polar; the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. . Chloride Ion And Water.
From learningschoolw1k5x.z21.web.core.windows.net
Diagram Of Ionic Bonding In Sodium Chloride Chloride Ion And Water Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. the chloride ion (cl−) is a type of anion which. Chloride Ion And Water.
From socratic.org
What is the chemical equation for HCl dissolving into water and Chloride Ion And Water the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. nonpolar molecules such as those found in grease or oil do not dissolve in water. the most common type of water in which chloride is the dominant anion is one in which sodium. Chloride Ion And Water.
From www.researchgate.net
Curves of adsorption of potassium ion, chloride ion, and water Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the chloride ion (cl−) is a type of anion which is commonly found. Chloride Ion And Water.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Chloride Ion And Water Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the most common type of water in which chloride is the. Chloride Ion And Water.
From www.alamy.com
How does sodium chloride (NaCl) dissolve in water? The Na ions attract Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. water can dissolve salt because the positive part of water molecules attracts the. Chloride Ion And Water.
From chemistry.stackexchange.com
ions Behavior of NaCl in water with electrodes Chemistry Stack Exchange Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. It has both a positive and a negative end. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. the most common type. Chloride Ion And Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Chloride Ion And Water We will first examine the process that occurs when. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. water can. Chloride Ion And Water.
From www.chegg.com
Solved Sodium and chloride ions and water are attracted to Chloride Ion And Water It has both a positive and a negative end. We will first examine the process that occurs when. this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines. Chloride Ion And Water.
From rwu.pressbooks.pub
Chapter 2. The Chemical Context of Life Introduction to Molecular and Chloride Ion And Water water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. nonpolar molecules such as those found in grease or oil do not dissolve. Chloride Ion And Water.
From www.dreamstime.com
Sodium chloride + water stock image. Image of base, chemicals 45367255 Chloride Ion And Water the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. water is attracted to the sodium chloride crystal because water. Chloride Ion And Water.
From www.numerade.com
SOLVED In an aqueous saltwater solution, the forces between sodium Chloride Ion And Water nonpolar molecules such as those found in grease or oil do not dissolve in water. water is attracted to the sodium chloride crystal because water is polar; We will first examine the process that occurs when. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Chloride Ion And Water.
From courses.lumenlearning.com
Why Life Depends on Water Biology for NonMajors I Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. nonpolar molecules such as those found in grease or oil do. Chloride Ion And Water.
From www.slideserve.com
PPT Unit 11 Liquids, Solids, and Solutions PowerPoint Presentation Chloride Ion And Water It has both a positive and a negative end. We will first examine the process that occurs when. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. water is attracted to the sodium chloride crystal because water is polar; nonpolar molecules such as those. Chloride Ion And Water.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chloride Ion And Water nonpolar molecules such as those found in grease or oil do not dissolve in water. It has both a positive and a negative end. We will first examine the process that occurs when. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. . Chloride Ion And Water.
From masterconceptsinchemistry.com
How does sodium chloride (NaCl) dissolve in water? Chloride Ion And Water water is attracted to the sodium chloride crystal because water is polar; the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. . Chloride Ion And Water.
From mydiagram.online
[DIAGRAM] Water And Sodium Chloride Molecular Diagram Chloride Ion And Water nonpolar molecules such as those found in grease or oil do not dissolve in water. water is attracted to the sodium chloride crystal because water is polar; the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. the most common type of. Chloride Ion And Water.
From www.getbodysmart.com
Ions Cations and Anions GetBodySmart Chloride Ion And Water water is attracted to the sodium chloride crystal because water is polar; the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. . Chloride Ion And Water.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Chloride Ion And Water Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. We will first examine the process that occurs when. the most. Chloride Ion And Water.
From courses.lumenlearning.com
Compounds Essential to Human Functioning Anatomy and Chloride Ion And Water this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. We will first examine the process that occurs when. Waters of this type. Chloride Ion And Water.
From slcc.pressbooks.pub
5.2 Water’s Interactions with Other Molecules College Biology I Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. It has both a positive and a negative end. nonpolar molecules. Chloride Ion And Water.
From socratic.org
What is it called when sodium and chloride ions separate when dissolved Chloride Ion And Water nonpolar molecules such as those found in grease or oil do not dissolve in water. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. the most common type of water in which chloride is the dominant anion is one in which sodium. Chloride Ion And Water.
From mavink.com
Sodium Chloride Structure Chloride Ion And Water We will first examine the process that occurs when. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. water. Chloride Ion And Water.
From shaunmwilliams.com
Lecture 13 Presentation Chloride Ion And Water We will first examine the process that occurs when. water is attracted to the sodium chloride crystal because water is polar; Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. water can dissolve salt because the positive part of water molecules attracts. Chloride Ion And Water.
From www.essentialchemicalindustry.org
Chlorine Chloride Ion And Water water is attracted to the sodium chloride crystal because water is polar; the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. . Chloride Ion And Water.
From www.youtube.com
Chloride ion with hydration sphere005 YouTube Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. It has both a positive and a negative end. Waters of this type tend to range from dilute solutions influenced by rainfall near the ocean to brines near saturation with respect to sodium chloride. We will first. Chloride Ion And Water.
From www.chegg.com
Solved Experiment 8 Determination of chloride ion in water Chloride Ion And Water We will first examine the process that occurs when. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. nonpolar molecules such as those found in grease or oil do not dissolve in water. water is attracted to the sodium chloride crystal because water is. Chloride Ion And Water.
From www.youtube.com
13.1.2 Describe the reactions of chlorine and the chlorides referred to Chloride Ion And Water We will first examine the process that occurs when. the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. this user. Chloride Ion And Water.
From www.studyread.com
Difference between Ion and Atom in Points with Examples Chloride Ion And Water the most common type of water in which chloride is the dominant anion is one in which sodium is the predominant cation. It has both a positive and a negative end. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. the chloride ion (cl−). Chloride Ion And Water.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chloride Ion And Water water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water. the chloride ion (cl−) is a type of anion which is commonly found in the environment and has important physiological functions and industrial uses. the most common type of water in which chloride is the. Chloride Ion And Water.
From www.nagwa.com
Question Video Using the Water Solubility Rules to Determine Which Chloride Ion And Water this user absolutely insists that when a chloride ion is present in water (for example, when nacl n a c l dissolves in. nonpolar molecules such as those found in grease or oil do not dissolve in water. water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative. Chloride Ion And Water.