Calorimetric Approach . The change in temperature of the measuring. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is. To use calorimetric data to calculate enthalpy changes. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related.
from www.semanticscholar.org
To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. Explain the technique of calorimetry. Explain the technique of calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. The change in temperature of the measuring. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical calorimetry data. To use calorimetric data to calculate enthalpy changes. Calorimetry is used to measure amounts of heat transferred to or from a substance.
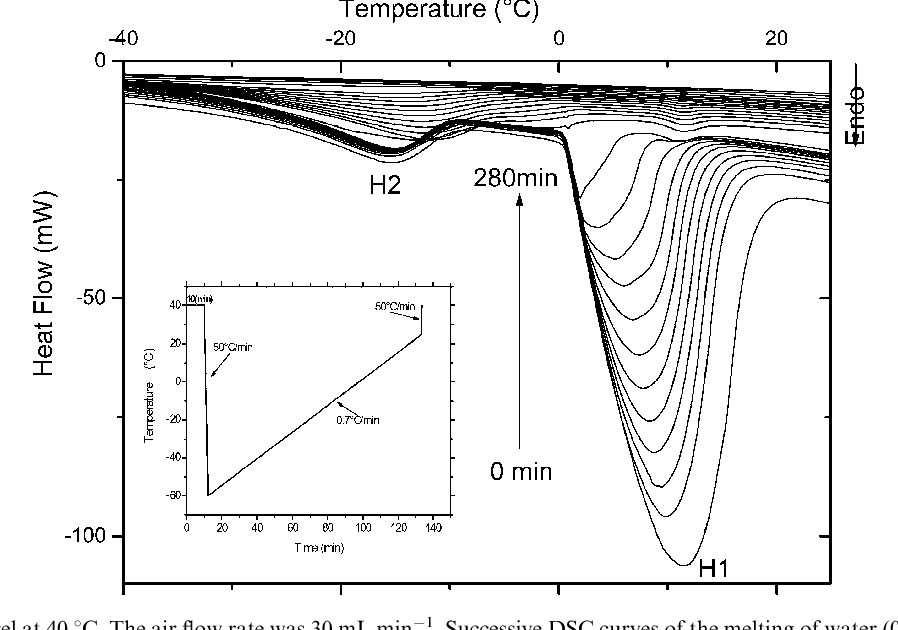
Figure 1 from Diffusion in mesoporous materials and polymers swelling
Calorimetric Approach One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. To use calorimetric data to calculate enthalpy changes. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. The change in temperature of the measuring. One technique we can use to measure the amount of heat involved in a chemical or physical process is. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical.
From www.researchgate.net
Schematic diagram of a calorimetric unit in the 2277 Thermal Activity Calorimetric Approach Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related.. Calorimetric Approach.
From www.researchgate.net
Calorimetric curves for CACGGBS mixtures with 0.4 of W/B ratio at an Calorimetric Approach Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. One technique we can use to measure the amount of heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change. Calorimetric Approach.
From www.semanticscholar.org
Figure 1 from A chipcalorimetric approach to the analysis of Ag Calorimetric Approach The change in temperature of the measuring. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). Explain the technique of calorimetry. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. To use calorimetric data to calculate. Calorimetric Approach.
From www.semanticscholar.org
Figure 2.1 from Probing the IonBinding Site of the Holliday Junction Calorimetric Approach Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical.. Calorimetric Approach.
From www.researchgate.net
(PDF) A noncalorimetric approach for investigating the moisture Calorimetric Approach Explain the technique of calorimetry. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. One technique we. Calorimetric Approach.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetric Approach Explain the technique of calorimetry. To use calorimetric data to calculate enthalpy changes. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. One technique we can use to measure the amount of heat involved in a. Calorimetric Approach.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetric Approach To do so, the heat is exchanged with a calibrated object (calorimeter). Explain the technique of calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calculate and interpret heat and related properties using typical calorimetry data. To use calorimetric data to calculate enthalpy changes. Explain the technique of calorimetry.. Calorimetric Approach.
From www.semanticscholar.org
Figure 1 from Diffusion in mesoporous materials and polymers swelling Calorimetric Approach One technique we can use to measure the amount of heat involved in a chemical or physical process is. One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. The change in temperature of the measuring. Calorimetry is used to measure amounts of heat transferred to or from. Calorimetric Approach.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetric Approach To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. One technique we can use to measure the amount of heat. Explain the technique of calorimetry. The change in temperature of the measuring. To use calorimetric data to calculate. Calorimetric Approach.
From www.researchgate.net
(PDF) The Mare Experiment. Calorimetric Approach to the Direct Calorimetric Approach Explain the technique of calorimetry. To use calorimetric data to calculate enthalpy changes. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. One technique we can use to measure the amount of heat involved in a chemical or. Calorimetric Approach.
From www.academia.edu
(PDF) Devolatilization and pyrolysis of refuse derived fuels Calorimetric Approach To use calorimetric data to calculate enthalpy changes. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount of heat involved in a chemical or. Calorimetric Approach.
From www.slideserve.com
PPT Power and Energy Measurements Chapters 39 and 42 PowerPoint Calorimetric Approach To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. To use calorimetric data to calculate enthalpy changes. One technique we can use to measure the amount of heat.. Calorimetric Approach.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetric Approach To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount of. Calorimetric Approach.
From www.researchgate.net
Traditional Calorimetric Methods Download Scientific Diagram Calorimetric Approach One technique we can use to measure the amount of heat involved in a chemical or physical process is. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and. Calorimetric Approach.
From www.academia.edu
(PDF) Calorimetric glass transition in a meanfield theory approach Calorimetric Approach Explain the technique of calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). To use calorimetric data to calculate enthalpy changes. The change in temperature of the measuring. To have any. Calorimetric Approach.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetric Approach To use calorimetric data to calculate enthalpy changes. Explain the technique of calorimetry. One technique we can use to measure the amount of heat. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount. Calorimetric Approach.
From www.researchgate.net
Calorimetric test set up. Download Scientific Diagram Calorimetric Approach Explain the technique of calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat. The change in temperature of the measuring. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calculate and interpret heat and related. Calorimetric Approach.
From www.researchgate.net
Simulation and measurement results (calorimetric approach ) of the Calorimetric Approach To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure amounts of heat transferred to or from a substance. The change in temperature of the measuring. Calorimetry, a vital. Calorimetric Approach.
From www.researchgate.net
Differential scanning calorimetric trace for PP film used in the study Calorimetric Approach Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical calorimetry data. The change in temperature of the measuring. To use calorimetric data to calculate enthalpy changes. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. One technique we can. Calorimetric Approach.
From www.researchgate.net
Simulation results (calorimetric approach) of the heater voltage U H as Calorimetric Approach To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry.. Calorimetric Approach.
From www.researchgate.net
Simulation results (calorimetric approach) of the heater voltage U H as Calorimetric Approach Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. To use calorimetric data to calculate enthalpy changes. One technique we can. Calorimetric Approach.
From www.mdpi.com
Batteries Free FullText A Generic Approach to Simulating Calorimetric Approach Explain the technique of calorimetry. To use calorimetric data to calculate enthalpy changes. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. To do so,. Calorimetric Approach.
From www.researchgate.net
A) Calorimetric thermogram at 10°C/min of the PP matrix of commingled Calorimetric Approach One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). Explain the technique of calorimetry. To have any meaning, the quantity that is actually measured. Calorimetric Approach.
From www.researchgate.net
Calorimetric curves for CACGGBS mixtures with 0.4 of W/B ratio at an Calorimetric Approach Explain the technique of calorimetry. Explain the technique of calorimetry. To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. Calculate and interpret heat and related properties using typical calorimetry data. To use calorimetric data to calculate enthalpy changes. The change in temperature of the. Calorimetric Approach.
From www.researchgate.net
(PDF) Neutron detection by measuring capture gammas in a calorimetric Calorimetric Approach One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. To do so, the heat is exchanged with a calibrated object (calorimeter). To use calorimetric data to calculate enthalpy changes. To have. Calorimetric Approach.
From www.researchgate.net
Calorimetric traces associated with curing of epoxy at different Calorimetric Approach Explain the technique of calorimetry. To use calorimetric data to calculate enthalpy changes. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange. Calorimetric Approach.
From documents.pub
Calorimetric approach for 3D dosimetry of high intensity therapeutic Calorimetric Approach Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. The change in temperature of the measuring. One technique we can use to measure the amount of heat. Explain the technique of calorimetry. Explain the technique of calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a. Calorimetric Approach.
From www.annualreviews.org
Thermodynamics of ProteinLigand Interactions Calorimetric Approaches Calorimetric Approach Explain the technique of calorimetry. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data.. Calorimetric Approach.
From www.slideserve.com
PPT Electrical and calorimetric measurements and related software Calorimetric Approach To use calorimetric data to calculate enthalpy changes. One technique we can use to measure the amount of heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). Explain the technique of. Calorimetric Approach.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Calorimetric Approach To have any meaning, the quantity that is actually measured in a calorimetric experiment, the change in the temperature of the device, must be related. The change in temperature of the measuring. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat. To use calorimetric data to. Calorimetric Approach.
From www.researchgate.net
Differential scanning calorimetric curve of [(CH3)2NH2]2ZnBr4 Calorimetric Approach One technique we can use to measure the amount of heat. To use calorimetric data to calculate enthalpy changes. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry is used to measure amounts of heat transferred to or from a substance. Explain the technique of calorimetry. The change in. Calorimetric Approach.
From www.researchgate.net
Calorimetric curves on heating and cooling for SBN and CBN crystals Calorimetric Approach The change in temperature of the measuring. One technique we can use to measure the amount of heat. Calculate and interpret heat and related properties using typical calorimetry data. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical.. Calorimetric Approach.
From www.researchgate.net
Differential scanning calorimetric thermograms (A) and powder Xray Calorimetric Approach Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry, a vital technique in the realm of physical sciences, revolves around the measurement of heat exchange in chemical. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount of heat. To do so, the heat is exchanged with. Calorimetric Approach.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetric Approach Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount of heat. To use calorimetric data to calculate enthalpy changes. Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use. Calorimetric Approach.
From www.slideserve.com
PPT Calorimetric Investigation under High Pressure PowerPoint Calorimetric Approach Calculate and interpret heat and related properties using typical calorimetry data. The change in temperature of the measuring. Explain the technique of calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. One technique we can use to measure the amount of heat. Explain the technique of calorimetry. Calculate and. Calorimetric Approach.