Lead Hydroxide Plus Nitric Acid . Lead does not react with sulphuric acid, due to the passivated pbo surface. The balanced equation will appear. Lead (ii) nitrate can be. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Enter an equation of an ionic. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions.
from www.slideshare.net
Lead (ii) nitrate can be. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an equation of an ionic. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. The balanced equation will appear. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water.
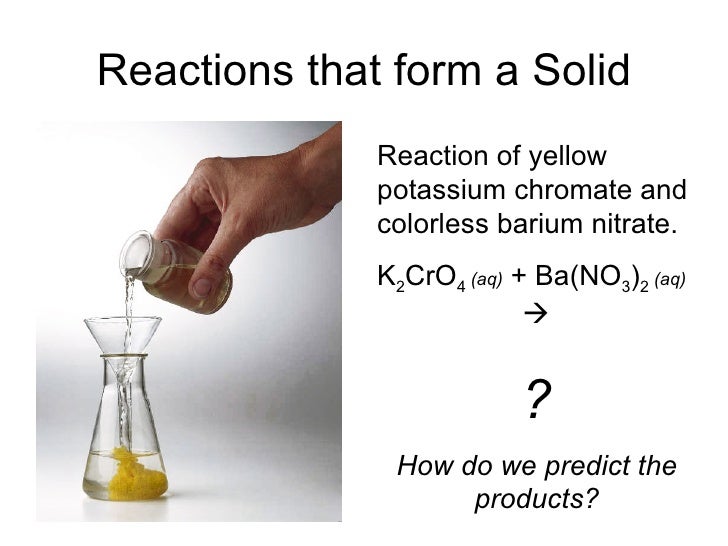
Chapter 8 Reactions in Aqueous Solution
Lead Hydroxide Plus Nitric Acid Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Lead (ii) nitrate can be. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead does not react with sulphuric acid, due to the passivated pbo surface. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Enter an equation of an ionic. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. The balanced equation will appear. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3.
From brainly.com
Write two equations for the neutralization of nitric acid, HNO, with Lead Hydroxide Plus Nitric Acid Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: The balanced equation will appear. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead (ii) nitrate can be. To balance a chemical equation, enter an equation. Lead Hydroxide Plus Nitric Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: The balanced equation will appear. Enter an equation of an ionic. Lead when reacted with nitric acid forms. Lead Hydroxide Plus Nitric Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Lead Hydroxide Plus Nitric Acid Lead (ii) nitrate can be. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Lead does not react with sulphuric acid, due to the passivated pbo surface. Experiments in this laboratory have shown that mixtures of. Lead Hydroxide Plus Nitric Acid.
From www.slideshare.net
Acids And Bases Lead Hydroxide Plus Nitric Acid Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Lead when reacted with. Lead Hydroxide Plus Nitric Acid.
From www.numerade.com
SOLVED The net ionic equation for the reaction of aqueous sodium Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for AgNO3 + FeCl3 = Fe(NO3)3 + AgCl Lead Hydroxide Plus Nitric Acid Lead does not react with sulphuric acid, due to the passivated pbo surface. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Enter. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
How to Balance NaNO3 + PbO = Pb(NO3)2 + Na2O YouTube Lead Hydroxide Plus Nitric Acid Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of. Lead Hydroxide Plus Nitric Acid.
From www.nagwa.com
Lesson Video Properties of Nitric Acid Nagwa Lead Hydroxide Plus Nitric Acid Lead (ii) nitrate can be. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of an ionic. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Lead (ii). Lead Hydroxide Plus Nitric Acid.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Lead Hydroxide Plus Nitric Acid Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead (ii) nitrate can be. Lead when reacted with nitric acid forms lead (ii) nitrate,. Lead Hydroxide Plus Nitric Acid.
From www.chemicals.co.uk
The Surprising Reaction Of Nitric Acid & Ammonia The Chemistry Blog Lead Hydroxide Plus Nitric Acid Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. The balanced equation will appear. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Enter an ionic equation (solubility states. Lead Hydroxide Plus Nitric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Hydroxide Plus Nitric Acid Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Enter an equation of an ionic. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Lead reacts slowly with hydrochloric acid,. Lead Hydroxide Plus Nitric Acid.
From pubs.acs.org
Reactions of Metals in Nitric Acid Writing Equations and Calculating Lead Hydroxide Plus Nitric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions. Lead Hydroxide Plus Nitric Acid.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write Lead Hydroxide Plus Nitric Acid Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the. Lead Hydroxide Plus Nitric Acid.
From www.slideshare.net
Acids And Bases Lead Hydroxide Plus Nitric Acid Lead (ii) nitrate can be. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Enter an. Lead Hydroxide Plus Nitric Acid.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Lead Hydroxide Plus Nitric Acid Enter an equation of an ionic. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance. Lead Hydroxide Plus Nitric Acid.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Lead Hydroxide Plus Nitric Acid Lead does not react with sulphuric acid, due to the passivated pbo surface. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
HNO3 + H2O (Nitric acid plus Water) YouTube Lead Hydroxide Plus Nitric Acid Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead. Lead Hydroxide Plus Nitric Acid.
From www.showme.com
Sodium Hydroxide and Nitric Acid Science ShowMe Lead Hydroxide Plus Nitric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: To balance a chemical equation, enter. Lead Hydroxide Plus Nitric Acid.
From www.animalia-life.club
Nitric Acid And Sulfuric Acid Lead Hydroxide Plus Nitric Acid Lead does not react with sulphuric acid, due to the passivated pbo surface. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. The balanced equation will appear. Lead (ii) nitrate can be. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. This page looks at the formation of some insoluble lead(ii) compounds. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Lead Hydroxide Plus Nitric Acid Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. The balanced equation will appear. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Lead (ii) chloride,. Lead Hydroxide Plus Nitric Acid.
From anhsanchem.com
Nitric Acid (Certified ACS Plus), Fisher Chemical Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. To balance a chemical equation, enter an equation of a chemical reaction and press. Lead Hydroxide Plus Nitric Acid.
From www.showme.com
ShowMe sodium carbonate and nitric acid Lead Hydroxide Plus Nitric Acid Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of an. Lead Hydroxide Plus Nitric Acid.
From sciencelab.co.ke
Lead Hydroxide Sciencelab limited Lead Hydroxide Plus Nitric Acid Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. To balance a chemical equation, enter an equation of a chemical. Lead Hydroxide Plus Nitric Acid.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. The balanced equation will appear. Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) nitrate can be. Experiments. Lead Hydroxide Plus Nitric Acid.
From www.chegg.com
Solved 5. lead(II) nitrate plus sodium sulfate b) c) 6. Lead Hydroxide Plus Nitric Acid Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an. Lead Hydroxide Plus Nitric Acid.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation ID4566566 Lead Hydroxide Plus Nitric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead does not react with sulphuric acid, due to the passivated pbo surface. Lead (ii) nitrate can be. The balanced equation will appear. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii). Lead Hydroxide Plus Nitric Acid.
From www.sciencephoto.com
Lead (II) hydroxide precipitate, 3 of 3 Stock Image C036/3123 Lead Hydroxide Plus Nitric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide, and ethylenediaminetetraacetic acid. Enter an equation of an ionic. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead reacts slowly with. Lead Hydroxide Plus Nitric Acid.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reactions that Lead Hydroxide Plus Nitric Acid Lead (ii) nitrate can be. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an equation of an ionic. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water.. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
Double displacement HCl + Pb(NO3)2 Hydrochloric acid + Lead(ii Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Lead (ii) chloride, a. Lead Hydroxide Plus Nitric Acid.
From www.transtutors.com
(Get Answer) The net ionic equation for the reaction between aqueous Lead Hydroxide Plus Nitric Acid To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead (ii) nitrate can be. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead when reacted with nitric acid forms lead (ii) nitrate, nitrogen oxide and water. Pb2 + (aq) + 2nh 3(aq) + 3h. Lead Hydroxide Plus Nitric Acid.
From www.showme.com
Lithium hydroxide and lead II nitrate Science ShowMe Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead (ii) nitrate can be. Enter an equation of an ionic. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Experiments in. Lead Hydroxide Plus Nitric Acid.
From www.tes.com
Metals and Acids Reactivity Series KS4 Edexcel 91 Teaching Resources Lead Hydroxide Plus Nitric Acid Enter an equation of an ionic. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. The balanced equation will appear. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: Experiments in this laboratory have shown that mixtures of fluoroboric acid, hydrogen peroxide,. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
AS/A Level ChemistryReaction of Lead Oxide and Nitric Acid. Calculate Lead Hydroxide Plus Nitric Acid Pb2 + (aq) + 2nh 3(aq) + 3h 2o(l) +. Lead (ii) nitrate can be. The balanced equation will appear. Lead does not react with sulphuric acid, due to the passivated pbo surface. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Enter an equation of an ionic. Experiments in this. Lead Hydroxide Plus Nitric Acid.
From www.youtube.com
Write the balanced chemical equation of the following word equation Lead Hydroxide Plus Nitric Acid This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead (ii) nitrate can be. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Enter an equation of an ionic. Lead (ii) ion. Lead Hydroxide Plus Nitric Acid.
From colouremployer8.gitlab.io
Sensational Balanced Equation Of Nitric Acid Gravitation Class 9 Lead Hydroxide Plus Nitric Acid Lead reacts slowly with hydrochloric acid, hcl and nitric acid, hno 3. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. Lead (ii) ion reacts with aqueous ammonia to precipitate a white basic salt, pb 2o(no 3) 2, rather than the expected lead (ii) hydroxide: This page looks. Lead Hydroxide Plus Nitric Acid.