What Happens When Zinc Is Added To Water . Does zinc react in distilled water? In each reaction, hydrogen gas is given off and the metal hydroxide is produced. The reactivity series of metals is a. In what way and in what form does zinc react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Elementary zinc does not react with water molecules. Quickly melts to form a ball; 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Observation when added to water: Burns violently with sparks and a lilac flame; Disappears rapidly, often with a. The ion does form a protective,. Most zinc today is obtained from zns,. All the alkali metals react vigorously with cold water. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal.
from www.teachoo.com
To soften permanent water, sodium carbonate (na 2 co 3) is added. Quickly melts to form a ball; Most zinc today is obtained from zns,. The ion does form a protective,. Disappears rapidly, often with a. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. Observation when added to water: Elementary zinc does not react with water molecules. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. All the alkali metals react vigorously with cold water.
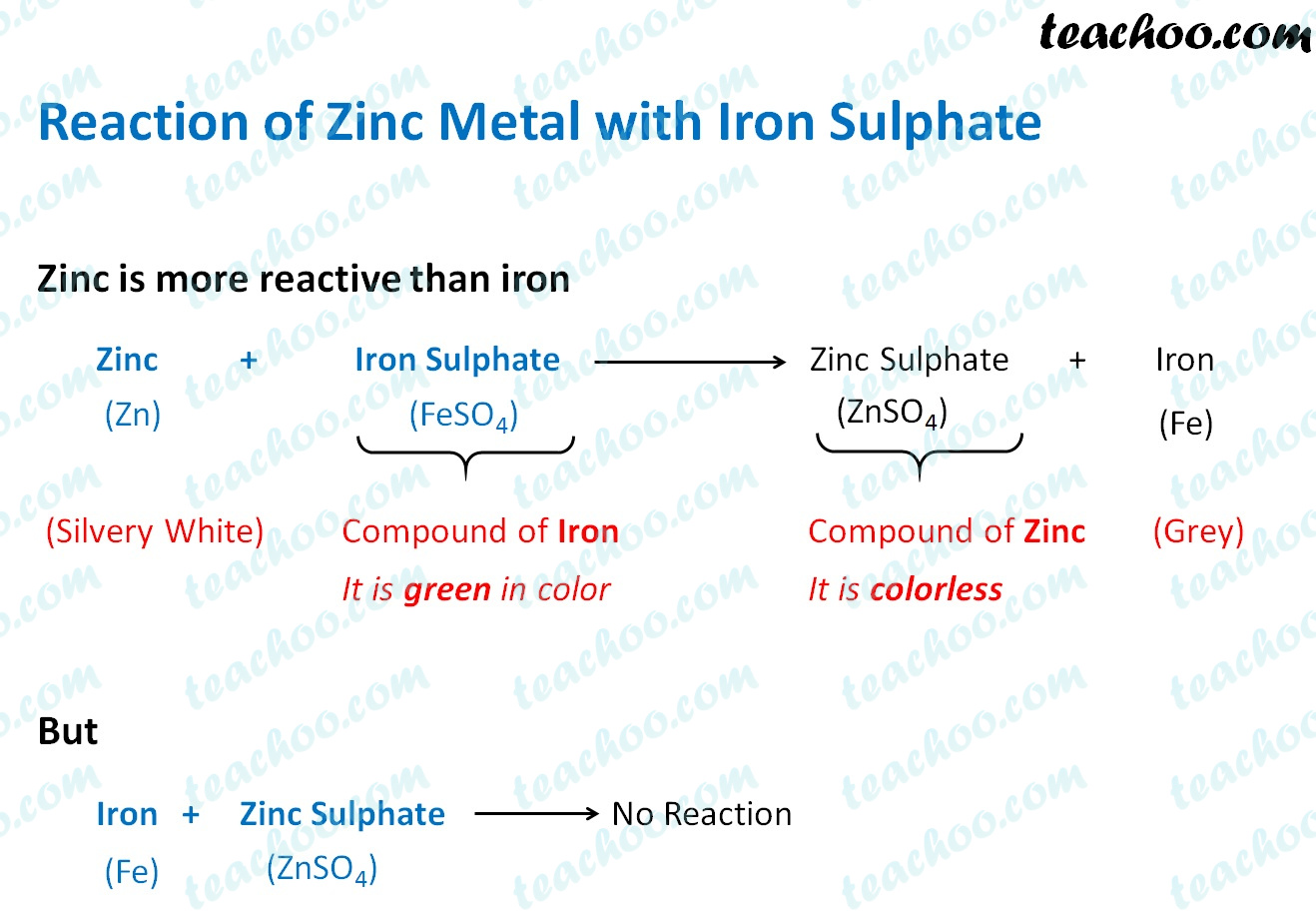
Displacement Reaction and Reactivity Series Concepts
What Happens When Zinc Is Added To Water The reactivity series of metals is a. The reactivity series of metals is a. All the alkali metals react vigorously with cold water. Does zinc react in distilled water? Elementary zinc does not react with water molecules. Disappears rapidly, often with a. The ion does form a protective,. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. Quickly melts to form a ball; In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Observation when added to water: Burns violently with sparks and a lilac flame; To soften permanent water, sodium carbonate (na 2 co 3) is added. In what way and in what form does zinc react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download What Happens When Zinc Is Added To Water Observation when added to water: The reactivity series of metals is a. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. Disappears rapidly, often with a. Does zinc react in distilled water? The ion does form a protective,. To soften permanent water, sodium carbonate (na 2 co 3) is added. All the alkali. What Happens When Zinc Is Added To Water.
From www.youtube.com
The reaction of Zinc(Zn) and Dilute Sulphuric Acid(H2SO4) YouTube What Happens When Zinc Is Added To Water Quickly melts to form a ball; To soften permanent water, sodium carbonate (na 2 co 3) is added. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Does zinc react in distilled water? All the alkali metals react vigorously with cold water. Burns violently with sparks and a lilac flame; All of group. What Happens When Zinc Is Added To Water.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 What Happens When Zinc Is Added To Water All the alkali metals react vigorously with cold water. Most zinc today is obtained from zns,. To soften permanent water, sodium carbonate (na 2 co 3) is added. Disappears rapidly, often with a. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Burns violently with sparks and a lilac flame;. What Happens When Zinc Is Added To Water.
From www.youtube.com
Zinc and Hydrochloric Acid YouTube What Happens When Zinc Is Added To Water Burns violently with sparks and a lilac flame; Quickly melts to form a ball; In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Elementary zinc does not react with water molecules. All the alkali metals react vigorously with cold water. Disappears rapidly, often with a. Does zinc react in distilled water? All of group 1. What Happens When Zinc Is Added To Water.
From www.slideshare.net
Replacement reactions What Happens When Zinc Is Added To Water Quickly melts to form a ball; Disappears rapidly, often with a. Most zinc today is obtained from zns,. Burns violently with sparks and a lilac flame; The ion does form a protective,. Does zinc react in distilled water? To soften permanent water, sodium carbonate (na 2 co 3) is added. All the alkali metals react vigorously with cold water. Observation. What Happens When Zinc Is Added To Water.
From www.youtube.com
Zinc Powder in water YouTube What Happens When Zinc Is Added To Water In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Elementary zinc does not react with water molecules. The reactivity series of metals is a. Disappears rapidly, often with a. To soften permanent water, sodium carbonate (na 2 co 3) is added. Quickly melts to form a ball; All of group 1 elements —lithium, sodium, potassium,. What Happens When Zinc Is Added To Water.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube What Happens When Zinc Is Added To Water To soften permanent water, sodium carbonate (na 2 co 3) is added. All the alkali metals react vigorously with cold water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Does zinc react in distilled water? In each reaction, hydrogen gas is given off and the metal hydroxide is produced.. What Happens When Zinc Is Added To Water.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts What Happens When Zinc Is Added To Water In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Does zinc react in distilled water? All the alkali metals react vigorously with cold water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Quickly melts to form a ball; Disappears rapidly, often with a. The. What Happens When Zinc Is Added To Water.
From slideplayer.com
What happens when substances are mixed with water ppt download What Happens When Zinc Is Added To Water Elementary zinc does not react with water molecules. Quickly melts to form a ball; All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Most zinc today is obtained from zns,. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Observation when added to. What Happens When Zinc Is Added To Water.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems What Happens When Zinc Is Added To Water The ion does form a protective,. In what way and in what form does zinc react with water? In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Burns violently with sparks and a lilac flame; Quickly melts to form a ball; Observation when added to water: All the alkali metals react vigorously with cold water.. What Happens When Zinc Is Added To Water.
From www.youtube.com
Zinc in Water Zincon Method Reagent preparation YouTube What Happens When Zinc Is Added To Water The ion does form a protective,. Does zinc react in distilled water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. All the alkali metals react vigorously with cold water. Elementary zinc does not react with water molecules. To soften permanent water, sodium carbonate (na 2 co 3) is added.. What Happens When Zinc Is Added To Water.
From brainly.in
Reaction of zinc with water Brainly.in What Happens When Zinc Is Added To Water Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. Does zinc react in distilled water? 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Disappears rapidly, often with a. The reactivity series of metals is a. Most zinc today is obtained from zns,. Observation when added. What Happens When Zinc Is Added To Water.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science What Happens When Zinc Is Added To Water Disappears rapidly, often with a. The ion does form a protective,. Most zinc today is obtained from zns,. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Quickly melts to form a ball; Elementary. What Happens When Zinc Is Added To Water.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free What Happens When Zinc Is Added To Water In what way and in what form does zinc react with water? Quickly melts to form a ball; Observation when added to water: In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Disappears rapidly, often with a. Does zinc react in distilled water? Sodium carbonate precipitates out the mg +2 and ca +2 ions out. What Happens When Zinc Is Added To Water.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube What Happens When Zinc Is Added To Water Does zinc react in distilled water? Quickly melts to form a ball; All the alkali metals react vigorously with cold water. To soften permanent water, sodium carbonate (na 2 co 3) is added. The reactivity series of metals is a. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. The ion does form. What Happens When Zinc Is Added To Water.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro What Happens When Zinc Is Added To Water The ion does form a protective,. In what way and in what form does zinc react with water? Quickly melts to form a ball; Observation when added to water: To soften permanent water, sodium carbonate (na 2 co 3) is added. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Elementary zinc does. What Happens When Zinc Is Added To Water.
From exojbgrtt.blob.core.windows.net
Zinc Facts And Uses at Sally Goodrich blog What Happens When Zinc Is Added To Water In each reaction, hydrogen gas is given off and the metal hydroxide is produced. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. The reactivity series of metals is a. All of group 1 elements —lithium, sodium,. What Happens When Zinc Is Added To Water.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental What Happens When Zinc Is Added To Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Disappears rapidly, often with a. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Quickly melts to form a ball;. What Happens When Zinc Is Added To Water.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq What Happens When Zinc Is Added To Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Most zinc today is obtained from zns,. Disappears rapidly, often with a. Elementary zinc does not react with water molecules. The reactivity series of metals is a. To soften permanent water, sodium carbonate (na 2 co 3) is added. The ion. What Happens When Zinc Is Added To Water.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0223 What Happens When Zinc Is Added To Water Does zinc react in distilled water? Observation when added to water: All the alkali metals react vigorously with cold water. To soften permanent water, sodium carbonate (na 2 co 3) is added. Most zinc today is obtained from zns,. In what way and in what form does zinc react with water? Elementary zinc does not react with water molecules. All. What Happens When Zinc Is Added To Water.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube What Happens When Zinc Is Added To Water Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. In what way and in what form does zinc react with water? Quickly melts to form a ball; 9 rows more reactive metals displace less reactive metals from their compounds and react with water. All the alkali metals react vigorously with cold water. Disappears. What Happens When Zinc Is Added To Water.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick What Happens When Zinc Is Added To Water In each reaction, hydrogen gas is given off and the metal hydroxide is produced. In what way and in what form does zinc react with water? Burns violently with sparks and a lilac flame; The ion does form a protective,. The reactivity series of metals is a. Elementary zinc does not react with water molecules. Does zinc react in distilled. What Happens When Zinc Is Added To Water.
From www.youtube.com
ZINC REACTION TO WATER YouTube What Happens When Zinc Is Added To Water Most zinc today is obtained from zns,. Disappears rapidly, often with a. All the alkali metals react vigorously with cold water. Observation when added to water: 9 rows more reactive metals displace less reactive metals from their compounds and react with water. In what way and in what form does zinc react with water? Sodium carbonate precipitates out the mg. What Happens When Zinc Is Added To Water.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate What Happens When Zinc Is Added To Water In each reaction, hydrogen gas is given off and the metal hydroxide is produced. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. The reactivity series of metals is a. Most zinc today is obtained from zns,. Elementary zinc does not react with water molecules. In what way and in what form does. What Happens When Zinc Is Added To Water.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When Zinc Is Added To Water Observation when added to water: All the alkali metals react vigorously with cold water. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. To soften permanent water, sodium carbonate (na 2 co 3) is added. Does zinc react in distilled water? Most zinc today is obtained from zns,. Quickly melts to form a. What Happens When Zinc Is Added To Water.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science What Happens When Zinc Is Added To Water The ion does form a protective,. All the alkali metals react vigorously with cold water. Quickly melts to form a ball; Burns violently with sparks and a lilac flame; 9 rows more reactive metals displace less reactive metals from their compounds and react with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. What Happens When Zinc Is Added To Water.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction What Happens When Zinc Is Added To Water Observation when added to water: 9 rows more reactive metals displace less reactive metals from their compounds and react with water. The reactivity series of metals is a. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Disappears rapidly, often with a. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously. What Happens When Zinc Is Added To Water.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube What Happens When Zinc Is Added To Water In each reaction, hydrogen gas is given off and the metal hydroxide is produced. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. The ion does form a protective,. Burns violently with sparks and a lilac flame; All the alkali metals react vigorously with cold water. In what way and in what form. What Happens When Zinc Is Added To Water.
From express.adobe.com
Zinc and Hydrochloric Acid What Happens When Zinc Is Added To Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Does zinc react in distilled water? Disappears rapidly, often. What Happens When Zinc Is Added To Water.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper What Happens When Zinc Is Added To Water Does zinc react in distilled water? The reactivity series of metals is a. All the alkali metals react vigorously with cold water. Disappears rapidly, often with a. Elementary zinc does not react with water molecules. To soften permanent water, sodium carbonate (na 2 co 3) is added. Burns violently with sparks and a lilac flame; All of group 1 elements. What Happens When Zinc Is Added To Water.
From www.youtube.com
What happens when zinc reacts with hot water YouTube What Happens When Zinc Is Added To Water Observation when added to water: The ion does form a protective,. Most zinc today is obtained from zns,. Burns violently with sparks and a lilac flame; To soften permanent water, sodium carbonate (na 2 co 3) is added. In what way and in what form does zinc react with water? 9 rows more reactive metals displace less reactive metals from. What Happens When Zinc Is Added To Water.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube What Happens When Zinc Is Added To Water Sodium carbonate precipitates out the mg +2 and ca +2 ions out as the respective metal. Observation when added to water: In each reaction, hydrogen gas is given off and the metal hydroxide is produced. The reactivity series of metals is a. To soften permanent water, sodium carbonate (na 2 co 3) is added. Disappears rapidly, often with a. Does. What Happens When Zinc Is Added To Water.
From zonebutterworthtb.z21.web.core.windows.net
Chemical Reactions With Zinc What Happens When Zinc Is Added To Water Elementary zinc does not react with water molecules. In what way and in what form does zinc react with water? Does zinc react in distilled water? The ion does form a protective,. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. Burns violently with sparks and a lilac flame; The reactivity series of metals is. What Happens When Zinc Is Added To Water.
From www.doubtnut.com
When dilute hydrochloric acid is added to granulated zinc placed i What Happens When Zinc Is Added To Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Quickly melts to form a ball; Burns violently with sparks and a lilac flame; 9 rows more reactive metals displace less reactive metals from their compounds and react with water. Does zinc react in distilled water? To soften permanent water, sodium. What Happens When Zinc Is Added To Water.
From www.youtube.com
What happens if you put zinc into aqua regia YouTube What Happens When Zinc Is Added To Water To soften permanent water, sodium carbonate (na 2 co 3) is added. Disappears rapidly, often with a. In each reaction, hydrogen gas is given off and the metal hydroxide is produced. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The ion does form a protective,. All the alkali metals. What Happens When Zinc Is Added To Water.