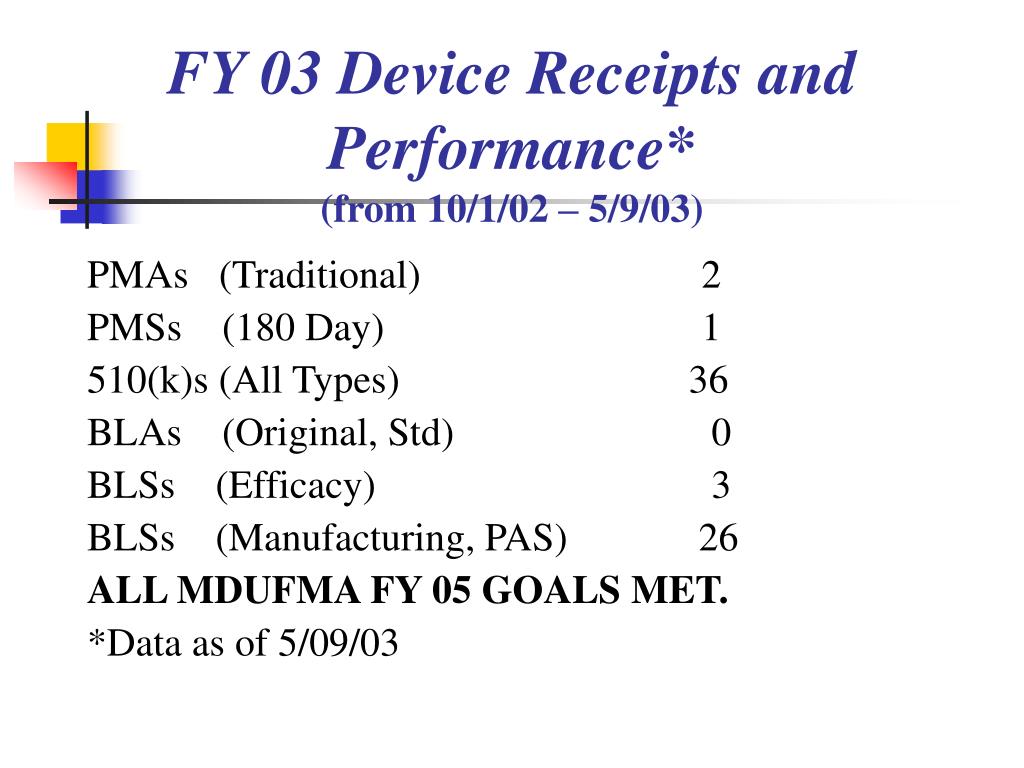
Medical Device User Fees . Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. On july 31, 2024, the u.s. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This letter provides information about the annual establishment registration fees, the small business program, and medical. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and.
from www.slideserve.com
This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. This letter provides information about the annual establishment registration fees, the small business program, and medical. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. On july 31, 2024, the u.s. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and.
PPT Medical Device User Fee and Modernization Act PowerPoint
Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. On july 31, 2024, the u.s. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This letter provides information about the annual establishment registration fees, the small business program, and medical.
From www.slideshare.net
Medical Device User Fee Amendments PDF Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides.. Medical Device User Fees.
From www.slideserve.com
PPT The Medical Device User Fee and Modernization Act of 2002 Medical Device User Fees User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list. Medical Device User Fees.
From orthospinenews.com
FDA raises medical device user fees more than 4 Ortho Spine News Medical Device User Fees This letter provides information about the annual establishment registration fees, the small business program, and medical. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. Food and drug administration (fda) issued a. Medical Device User Fees.
From www.rcainc.com
FDA Medical Device User Fees for Fiscal Years 20162017 Regulatory Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This letter provides information about the annual establishment registration fees, the small business program, and medical. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. This notice establishes the fee rates for fy 2022, which apply. Medical Device User Fees.
From digital.library.unt.edu
The FDA Medical Device User Fee Program MDUFA IV Reauthorization Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This letter provides information about the annual establishment registration fees, the small business program, and medical. On july 31, 2024, the u.s. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices,. Medical Device User Fees.
From operonstrategist.com
FDA Announces New Medical Device User Fee Amendments ( MDUFA ) for FY Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30,. Medical Device User Fees.
From www.slideserve.com
PPT Medical Device User Fee and Modernization Act PowerPoint Medical Device User Fees On july 31, 2024, the u.s. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This notice establishes the fee rates for fy 2025, which apply from october 1,. Medical Device User Fees.
From vantagemedtech.com
FDA Medical Device User Fees (MDUFA) for 2024 Vantage Medtech Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. This email provides information about the medical device user fees, annual establishment registration fees, and the small business.. Medical Device User Fees.
From everycrsreport.com
The FDA Medical Device User Fee Program MDUFA IV Reauthorization Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. User fees have. Medical Device User Fees.
From present5.com
Medical Device User Fee and Modernization Act of Medical Device User Fees On july 31, 2024, the u.s. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and. Medical Device User Fees.
From www.fdaimports.com
FDA Announces New and Updated Drug and Medical Device User Fees Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This letter provides information about the annual establishment registration fees, the small business program, and medical. On july 31, 2024, the. Medical Device User Fees.
From www.g2intelligence.com
Medical Device User Fees Agreement Finally Nearing the Finish Line G2 Medical Device User Fees User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This letter provides information about the annual establishment registration fees, the small business program, and medical. On july 31, 2024, the u.s. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and. Medical Device User Fees.
From www.jomakeitfda.com
Medical Device User Fee Rates for Fiscal Year 2023 Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. Under the user fee system, medical. Medical Device User Fees.
From www.provisionfda.com
FDA Medical Device User Fees Increase 7 for 2021 Fiscal Year Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. On july 31, 2024, the u.s. This letter provides information about the annual establishment registration fees, the small business program, and medical. This. Medical Device User Fees.
From www.slideserve.com
PPT The Medical Device User Fee and Modernization Act of 2002 Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This letter provides information about the annual establishment registration fees, the small business program, and medical. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. User fees have helped make. Medical Device User Fees.
From www.slideserve.com
PPT Medical Device User Fee and Modernization Act PowerPoint Medical Device User Fees On july 31, 2024, the u.s. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. User fees have helped make new human and animal drugs, generic human and animal drugs,. Medical Device User Fees.
From medicaldeviceacademy.com
Medical device 510k, quality systems, audits, and training Medical Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This letter provides information about the annual establishment registration fees, the small business program, and medical. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. On july. Medical Device User Fees.
From www.lachmanconsultants.com
FDA Announces MDUFA and Outsourcing User Fee Rates for FY 2020 All Medical Device User Fees On july 31, 2024, the u.s. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. Food and drug administration (fda) issued a federal register notice announcing medical. Medical Device User Fees.
From emmainternational.com
Understanding Medical Device User Fees Medical Device User Fees On july 31, 2024, the u.s. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. This letter provides information about the annual establishment registration fees, the small. Medical Device User Fees.
From www.slideserve.com
PPT The Medical Device User Fee and Modernization Act of 2002 Medical Device User Fees This letter provides information about the annual establishment registration fees, the small business program, and medical. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. On july 31, 2024, the u.s. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and. Medical Device User Fees.
From www.medicaldesignandoutsourcing.com
New medical device user fees agreement passes House MDO Medical Device User Fees This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their.. Medical Device User Fees.
From digital.library.unt.edu
Medical Device User Fees and User Fee Acts UNT Digital Library Medical Device User Fees User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This letter provides information about the annual establishment registration fees, the small business program, and medical. Food and drug administration. Medical Device User Fees.
From array.aami.org
FDA Medical Device User Fees Increase for 2024 AAMI News Medical Device User Fees Food and drug administration (fda) issued a federal register notice announcing medical device user fees. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. User fees have. Medical Device User Fees.
From medicaldeviceacademy.com
FDA User Fees 2020 Medical Device Academy Medical Device Academy Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. On july. Medical Device User Fees.
From www.provisionfda.com
Reminder FDA New Medical Device User Fees for FY 2023 Medical Device User Fees Food and drug administration (fda) issued a federal register notice announcing medical device user fees. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This letter provides information about the annual establishment registration fees, the small business program, and medical. User fees have helped make new human and. Medical Device User Fees.
From cosmereg.com
FDA 2023 Medical Device User Fees Announcement Cosmereg Medical Device User Fees On july 31, 2024, the u.s. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. Food and drug administration (fda) issued a federal register notice announcing medical device user fees.. Medical Device User Fees.
From www.provisionfda.com
FDA, FY 2024 Medical Device User Fees Notice Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This notice establishes. Medical Device User Fees.
From www.afpharmaservice.com
Medical Device User Fee Rates for Fiscal Year 2024 Medical Device User Fees User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. On july 31, 2024, the u.s. This notice establishes the fee rates for fy 2022, which apply from october 1,. Medical Device User Fees.
From knconsultingandservices.com
USFDA user fees for Medical Devices Medical Device Consulting Company Medical Device User Fees Food and drug administration (fda) issued a federal register notice announcing medical device user fees. This letter provides information about the annual establishment registration fees, the small business program, and medical. On july 31, 2024, the u.s. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This email. Medical Device User Fees.
From www.meditrial.net
US FDA publishes user fee amounts for medical device manufacturers in Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. Food and drug administration (fda) issued a federal register notice announcing medical device user fees. Under the user fee system, medical. Medical Device User Fees.
From medicaldeviceacademy.com
MDUFA IV FDA User Fee Increase Strategic Implications Medical Medical Device User Fees This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. On july 31, 2024, the u.s. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. User fees have helped make new human and animal drugs, generic human. Medical Device User Fees.
From medicaldeviceacademy.com
FDA User Fees 2021 Medical Device Academy Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This notice establishes the fee rates for fy 2022, which apply from october 1, 2021, through september 30, 2022, and provides. This email provides information about the medical device user fees, annual establishment registration fees, and the small business.. Medical Device User Fees.
From www.slideserve.com
PPT Medical Device User Fee Reauthorization & FDA Update PowerPoint Medical Device User Fees Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides.. Medical Device User Fees.
From www.slideserve.com
PPT Medical Devices Approval Process PowerPoint Presentation, free Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. This notice establishes the fee rates for fy 2025, which apply from october 1, 2024, through september 30, 2025, and provides. On july 31, 2024, the u.s. User fees have helped make new human and animal drugs, generic human and animal drugs,. Medical Device User Fees.
From www.provisionfda.com
FDA, FY 2024 Medical Device User Fees Notice Medical Device User Fees This email provides information about the medical device user fees, annual establishment registration fees, and the small business. Under the user fee system, medical device companies pay fees to the fda when they register their establishments and list their. User fees have helped make new human and animal drugs, generic human and animal drugs, medical devices, biologics, and. This notice. Medical Device User Fees.