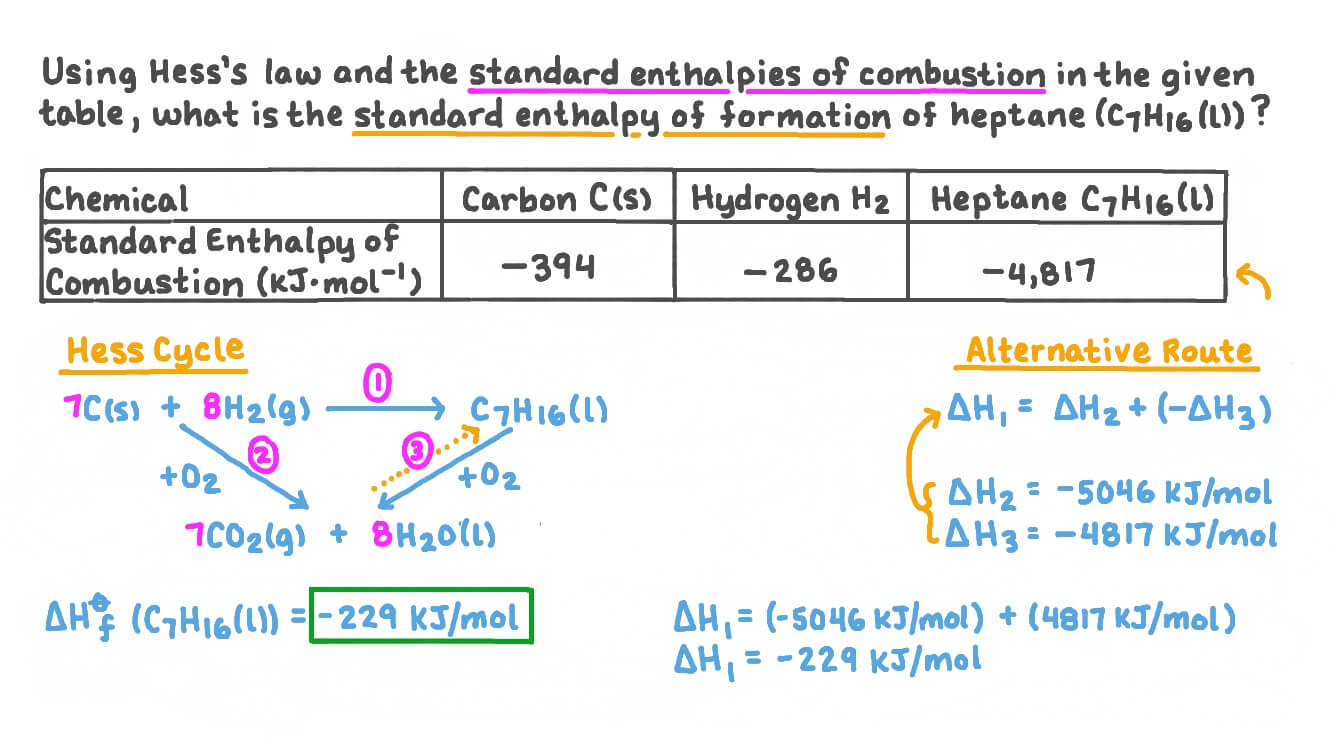
Standard Enthalpy Of Formation Is Negative . The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition.
from www.nagwa.com
In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c.
Question Video Calculating the Standard Enthalpy of Formation for
Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in. Standard Enthalpy Of Formation Is Negative.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Is Negative.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in. Standard Enthalpy Of Formation Is Negative.
From www.chegg.com
Solved Calculate the Standard Enthalpy of Formation of NOCl Standard Enthalpy Of Formation Is Negative 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. A standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Enthalpy Of Formation Is Negative.
From www.chemistrystudent.com
Enthalpy Changes (ALevel) ChemistryStudent Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol δfho. Standard Enthalpy Of Formation Is Negative.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID1204656 Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. By. Standard Enthalpy Of Formation Is Negative.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. In general, any enthalpy of reaction is the measure of the energy. Standard Enthalpy Of Formation Is Negative.
From www.w3schools.blog
Standard enthalpy of ionization W3schools Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c.. Standard Enthalpy Of Formation Is Negative.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. The standard enthalpy of formation of any element in its standard state. Standard Enthalpy Of Formation Is Negative.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3. Standard Enthalpy Of Formation Is Negative.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. The standard enthalpy of formation is given the symbol δfho δ f h o, where. Standard Enthalpy Of Formation Is Negative.
From www.chemistrysteps.com
Energy and Organic Chemistry Reactions Chemistry Steps Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Is Negative.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Standard Enthalpy Of Formation Is Negative.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Is Negative.
From slideplayer.com
Enthalpy of formation ΔHºf ppt download Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Is Negative.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o),. Standard Enthalpy Of Formation Is Negative.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol δfho δ f h. Standard Enthalpy Of Formation Is Negative.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. In general, any enthalpy of reaction is the measure of the. Standard Enthalpy Of Formation Is Negative.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Is Negative 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. By definition, the standard enthalpy of formation of an element in its most stable form is. Standard Enthalpy Of Formation Is Negative.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Is Negative By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as. Standard Enthalpy Of Formation Is Negative.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is a. Standard Enthalpy Of Formation Is Negative.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb Standard Enthalpy Of Formation Is Negative For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of. Standard Enthalpy Of Formation Is Negative.
From study.com
How to Draw & Label Enthalpy Diagrams Lesson Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. A standard enthalpy of formation $δh°_f$ is an enthalpy change for. Standard Enthalpy Of Formation Is Negative.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2. Standard Enthalpy Of Formation Is Negative.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Enthalpy Of Formation Is Negative.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2. Standard Enthalpy Of Formation Is Negative.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Is Negative A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation Is Negative.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Is Negative In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is given the symbol δfho δ f. Standard Enthalpy Of Formation Is Negative.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Standard Enthalpy Of Formation Is Negative.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Is Negative In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By definition, the standard enthalpy of formation of an element in its most stable form is. Standard Enthalpy Of Formation Is Negative.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. A standard enthalpy. Standard Enthalpy Of Formation Is Negative.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Is Negative For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is the most stable form at 1 atm pressure and 25°c. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. By definition, the standard enthalpy. Standard Enthalpy Of Formation Is Negative.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Standard Enthalpy Of Formation Is Negative.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation Is Negative.
From www.slideserve.com
PPT Enthalpy Changes PowerPoint Presentation, free download ID2914427 Standard Enthalpy Of Formation Is Negative The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. In general, any enthalpy of reaction is the measure of the energy remainder after breaking the bonds (positive) and what energy. The standard enthalpy of formation of any element in its standard state is zero by. Standard Enthalpy Of Formation Is Negative.