Assay Of Product . Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption.
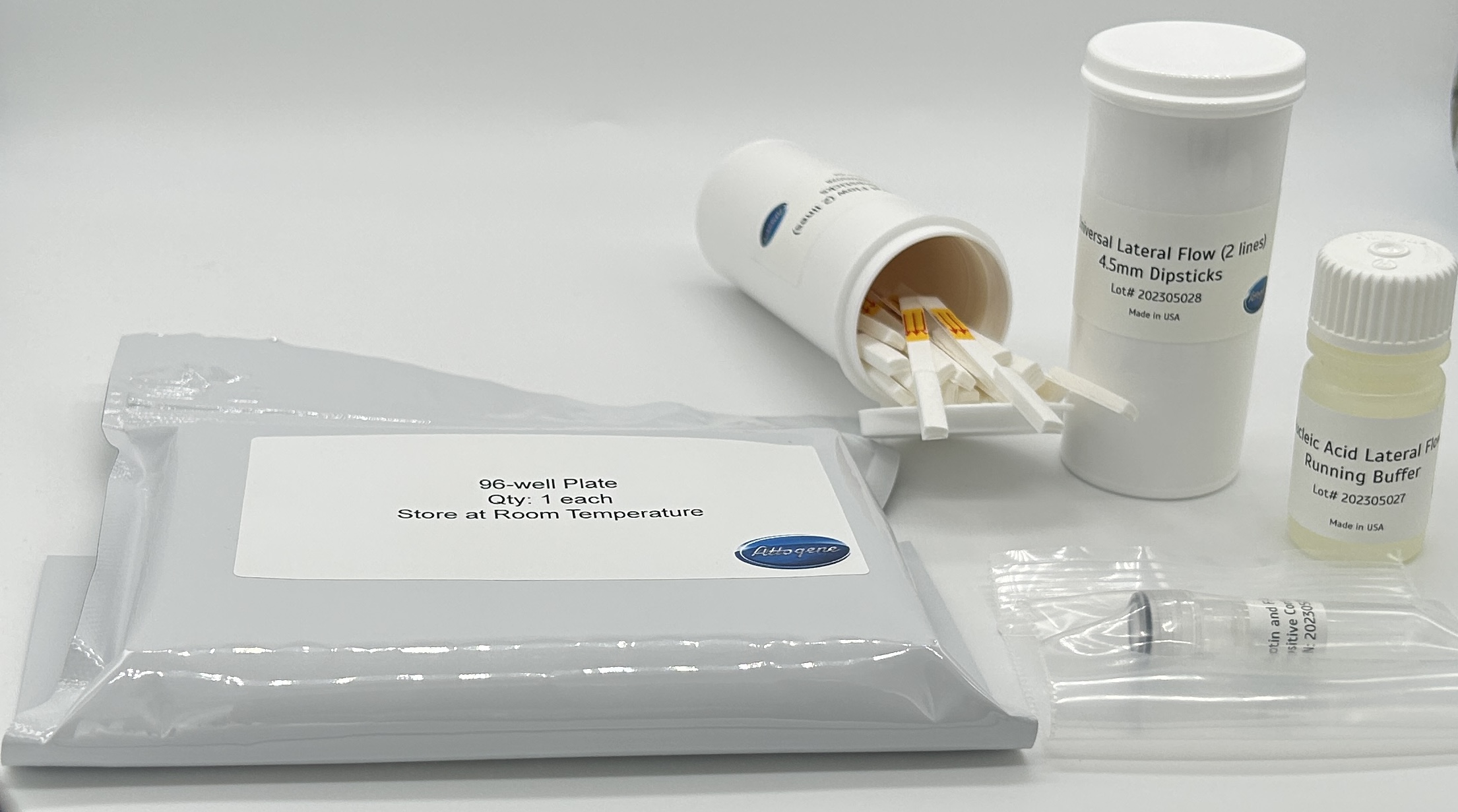
from www.attogene.com
Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); Test procedures and acceptance criteria for new drug substances and new drug products: During product development, manufacturing process evaluation, or commercial product release and stability testing, an. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products.
Universal Lateral Flow Assay Kit (Gold) Attogene
Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. During product development, manufacturing process evaluation, or commercial product release and stability testing, an. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity);
From www.megazyme.com
AlphaAmylase Assay Kit Measurement AlphaAmylase ceralpha Megazyme Assay Of Product Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. An assay is a laboratory procedure or test that. Assay Of Product.
From assaypro.com
About Us Assaypro Assay Of Product Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Test procedures and acceptance criteria for new drug substances and new drug products: Assay of active substances (and also for. Assay Of Product.
From www.megazyme.com
Catalase Assay Kit Megazyme Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Test procedures and acceptance criteria for new drug substances and new. Assay Of Product.
From www.researchgate.net
Assay Procedure overview of MATS ELISA. Various steps of the assay Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Nelson labs performs drug assays for active pharmaceutical ingredients and drug. Assay Of Product.
From www.thermofisher.com
Pierce LDH Cytotoxicity Assay Kit Thermo Fisher Scientific Assay Of Product Test procedures and acceptance criteria for new drug substances and new drug products: Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques,. Assay Of Product.
From www.fishersci.com
InvitrogenCyQUANT LDH Cytotoxicity Assay 1000 assaysCell Analysis Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Test procedures and acceptance criteria for new drug substances and new drug products: Assay of active substances (and also for herbal. Assay Of Product.
From www.fishersci.fi
Thermo Scientific™ Pierce™ BCA Protein Assay Kit Reducing Agent Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Nelson labs performs. Assay Of Product.
From www.britannica.com
Enzymelinked immunosorbent assay (ELISA) Definition, Uses, & Method Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Test procedures and acceptance criteria for new drug substances and new drug products: Chapters 9, 10, and 11 present general. Assay Of Product.
From childhealthpolicy.vumc.org
💌 Essay about product. Introducing New Product, Essay Example. 20221007 Assay Of Product An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Assay of active substances (and also for herbal medicines, quantitative determination. Assay Of Product.
From www.frontiersin.org
Frontiers AutoPlate Rapid DoseResponse Curve Analysis for Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Test procedures and. Assay Of Product.
From www.thermofisher.com
Pierce BCA Protein Assay Kit Thermo Fisher Scientific Assay Of Product An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Test procedures and acceptance criteria for new drug substances and new drug products: Chapters 9, 10, and. Assay Of Product.
From www.bio-techne.com
Multiplex Cytokine Assay Kits Products BioTechne Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Assay of active substances (and also. Assay Of Product.
From studymoose.com
Market Research Method of Selling a Product Free Essay Example Assay Of Product During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Test procedures and acceptance criteria for new. Assay Of Product.
From www.scribd.com
A Study On Marketing Mix of Nestle Product PDF Analysis Nestlé Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of. Assay Of Product.
From pharmaguddu.com
Understanding Drug Product Assay and Potency » Pharmaguddu Assay Of Product During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component. Assay Of Product.
From phdessay.com
How We Market your Products Assay Of Product During product development, manufacturing process evaluation, or commercial product release and stability testing, an. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures. Assay Of Product.
From cvloadzone.web.fc2.com
Product Review Essay Examples Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. During product development, manufacturing process evaluation, or commercial product release and stability. Assay Of Product.
From labplan.ie
Automating Cell Based Assays Labplan Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); During product development,. Assay Of Product.
From catalog.invivoscribe.com
NPM1 MRD Assay Invivoscribe Assay Of Product During product development, manufacturing process evaluation, or commercial product release and stability testing, an. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component. Assay Of Product.
From diapharma.com
QuickZyme Total Protein Assay Kit DiaPharma Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Chapters 9, 10, and 11 present general. Assay Of Product.
From www.cephamls.com
Bradford Protein Assay with BSA Protein Standard Cepham Life Sciences Assay Of Product During product development, manufacturing process evaluation, or commercial product release and stability testing, an. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Test procedures and acceptance criteria for new drug substances and new drug products: Chapters 9, 10, and. Assay Of Product.
From ainulizzatti.weebly.com
Protein Assay Powerangerspink Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. During product development, manufacturing process evaluation, or commercial. Assay Of Product.
From www.researchgate.net
Comparison of various cellular immunity assays. Each assay is compared Assay Of Product Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. It provides guidance on the setting and justification of acceptance criteria and the. Assay Of Product.
From www.bio-rad.com
Protein Assay Kits and Cuvettes Life Science Research BioRad Assay Of Product Test procedures and acceptance criteria for new drug substances and new drug products: An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug. Assay Of Product.
From www.fishersci.at
Thermo Scientific™ Pierce™ BCA Protein Assay Kit BCA Protein Assay Kit Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. An assay is a laboratory procedure or. Assay Of Product.
From studylib.net
Assay Design tips dr.. Assay Of Product Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. During product development, manufacturing process evaluation, or. Assay Of Product.
From www.studocu.com
Detection AND Assay OF Fermentation Products DETECTION AND ASSAY OF Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Test. Assay Of Product.
From www.attogene.com
Universal Lateral Flow Assay Kit (Gold) Attogene Assay Of Product An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Test procedures and acceptance criteria for new drug substances and new drug. Assay Of Product.
From phdessay.com
Product Design Essay Assay Of Product During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such. Assay Of Product.
From www.megazyme.com
Hydrogen Peroxide Assay Kit (Megaplex Red) for analysis of hydrogen Assay Of Product An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. During product development, manufacturing process evaluation, or commercial product release and stability testing, an. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic. Assay Of Product.
From www.researchgate.net
NBT reduction assay showing an increase in ROS amount in the treated Assay Of Product Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. Test procedures and acceptance criteria for new drug substances and new. Assay Of Product.
From www.genengnews.com
Potency Assays for Cell and Gene Therapy Assay Of Product Nelson labs performs drug assays for active pharmaceutical ingredients and drug products using techniques, such as hlpc, gc, and absorption. Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); Test procedures and acceptance criteria for new drug substances and new drug products: An assay is a laboratory procedure or test that. Assay Of Product.
From technicalcollege.web.fc2.com
Enzyme assays Assay Of Product Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Test procedures and acceptance criteria for new drug substances and new drug products: It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. Assay of active substances (and also for herbal. Assay Of Product.
From www.thermofisher.com
Pierce 660nm Protein Assay Kit Thermo Fisher Scientific Assay Of Product Assay of active substances (and also for herbal medicines, quantitative determination of the constituents with known therapeutic activity); Chapters 9, 10, and 11 present general concepts of assay and impurity analysis of drug substances and drug products. Test procedures and acceptance criteria for new drug substances and new drug products: Nelson labs performs drug assays for active pharmaceutical ingredients and. Assay Of Product.
From www.deltaclon.com
Deltaclon > Producto > AssaySense Human Urokinase (uPA) Chromogenic Assay Of Product It provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug. An assay is a laboratory procedure or test that is performed to determine the presence, concentration, quality, or biological activity of a specific substance or component within a sample. During product development, manufacturing process evaluation, or commercial product release and. Assay Of Product.