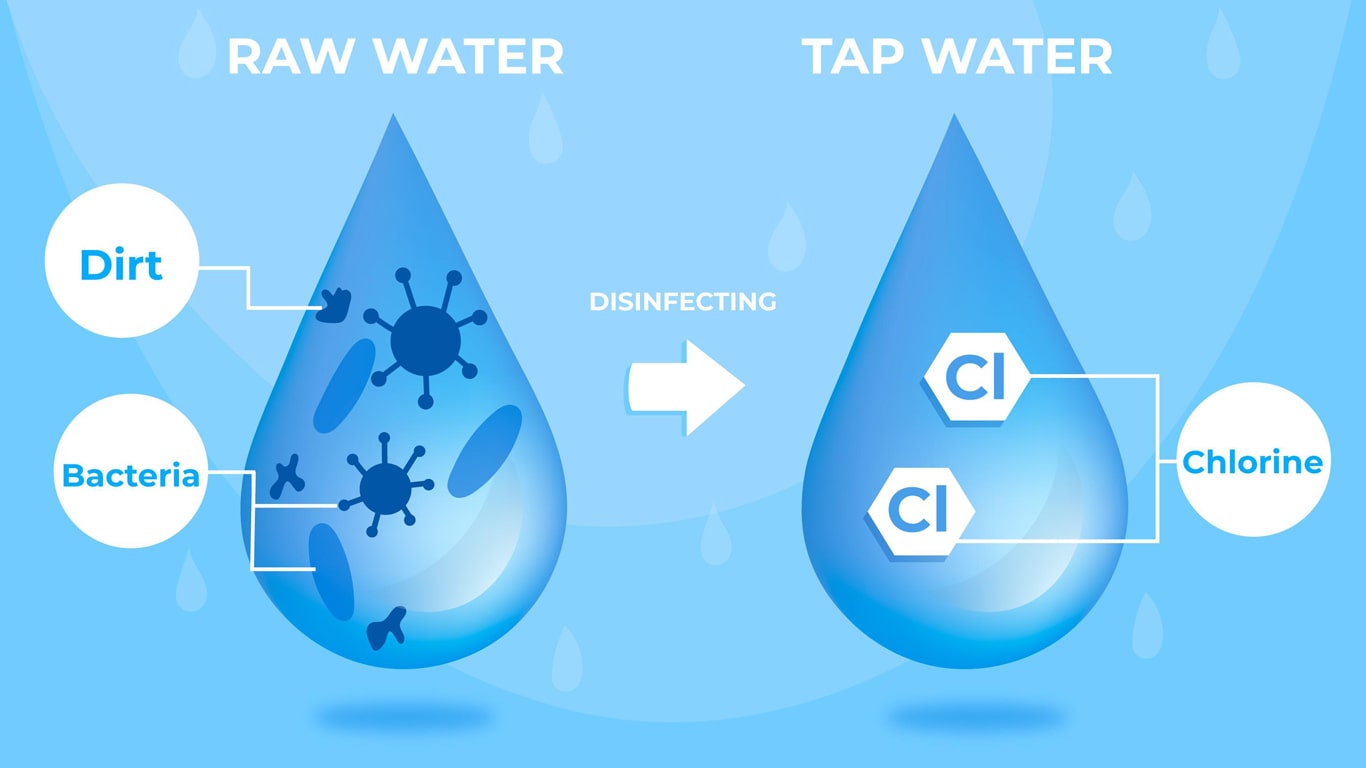
Chlorine In Water Equation . chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. chlorine is available in three forms: chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. Includes kit list and safety instructions. The chlorine will react with organic. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. when chlorine enters water, it immediately begins to react with compounds found in the water. the dose can be determined by: generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical.
from www.techquintal.com
generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. chlorine is available in three forms: chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. when chlorine enters water, it immediately begins to react with compounds found in the water. Includes kit list and safety instructions. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). the dose can be determined by: revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. The chlorine will react with organic.
10 Advantages and Disadvantages of Chlorination of Water to Know Tech
Chlorine In Water Equation chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. The chlorine will react with organic. the dose can be determined by: Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. Includes kit list and safety instructions. when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is available in three forms: chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride In Water Equation Chlorine In Water Equation chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. Includes kit list and safety instructions. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. The chlorine will react with organic. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they. Chlorine In Water Equation.
From www.alamy.com
Chlorine dioxide molecule hires stock photography and images Alamy Chlorine In Water Equation Includes kit list and safety instructions. The chlorine will react with organic. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite. Chlorine In Water Equation.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Chlorine In Water Equation chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. when chlorine enters water, it immediately begins to react with compounds found in the water. the. Chlorine In Water Equation.
From www.techquintal.com
10 Advantages and Disadvantages of Chlorination of Water to Know Tech Chlorine In Water Equation The chlorine will react with organic. Includes kit list and safety instructions. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. the dose can be determined by: when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is. Chlorine In Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Equation For Hydrogen Chloride In Water Chlorine In Water Equation generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. The chlorine will react with organic. Chlorine dose, mg/l = chlorine demand, mg/l +. Chlorine In Water Equation.
From exogwagyf.blob.core.windows.net
Chlorine And Acid at Charles Borkowski blog Chlorine In Water Equation the dose can be determined by: generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. when chlorine enters water, it immediately. Chlorine In Water Equation.
From www.numerade.com
SOLVED The equation for dissolving sodium chloride in water is given Chlorine In Water Equation the dose can be determined by: when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. The chlorine will react with organic. Chlorine dose, mg/l = chlorine. Chlorine In Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Gaseous Hydrogen Chloride Is Dissolved In Water Equation Chlorine In Water Equation Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). the dose can be determined by: The chlorine will react with organic. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. Includes kit list and safety instructions. when chlorine enters water, it immediately begins to react with compounds found in. Chlorine In Water Equation.
From www.slideserve.com
PPT Residual Chlorine & Chlorine Demand PowerPoint Presentation ID Chlorine In Water Equation revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Includes kit list and safety instructions. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. chlorine is only slightly soluble. Chlorine In Water Equation.
From es.slideshare.net
Determination of chloride content in water by Volhard method Chlorine In Water Equation chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. the dose can be determined by:. Chlorine In Water Equation.
From fity.club
Chlorine Gas Formula Chlorine In Water Equation The chlorine will react with organic. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is added to raw water to eliminate algae and. Chlorine In Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Equation For Hydrogen Chloride In Water Chlorine In Water Equation when chlorine enters water, it immediately begins to react with compounds found in the water. The chlorine will react with organic. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. . Chlorine In Water Equation.
From www.tessshebaylo.com
Equation For Dissolving Ammonium Chloride In Water Tessshebaylo Chlorine In Water Equation chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. The chlorine will react with organic. Includes kit list and safety instructions. when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is available in. Chlorine In Water Equation.
From www.youtube.com
Equation for LiCl + H2O (Lithium chloride + Water) YouTube Chlorine In Water Equation revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t. Chlorine In Water Equation.
From dxosibdrh.blob.core.windows.net
Chlorine In Water Treatment Equation at Charles Patrick blog Chlorine In Water Equation Includes kit list and safety instructions. The chlorine will react with organic. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. revision. Chlorine In Water Equation.
From www.youtube.com
How to Balance Cl2 + H2O = HCl + O2 (Chlorine gas + Water) YouTube Chlorine In Water Equation revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. when chlorine enters water, it immediately begins to react with compounds found in the water. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. Includes kit list and safety instructions. chlorine is added to. Chlorine In Water Equation.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride In Water Equation Chlorine In Water Equation Includes kit list and safety instructions. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. when chlorine enters water, it immediately begins to react with compounds found in the water. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. . Chlorine In Water Equation.
From waterfilterguru.com
Chloride in Water Chlorine In Water Equation Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. Includes kit list and safety instructions. The chlorine will react with organic. . Chlorine In Water Equation.
From www.youtube.com
13.1.2 Describe the reactions of chlorine and the chlorides referred to Chlorine In Water Equation The chlorine will react with organic. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. chlorine is available in three forms: generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. the dose can be determined by: revision notes on. Chlorine In Water Equation.
From www.youtube.com
How to Write the Formula for Hydrogen chloride YouTube Chlorine In Water Equation chlorine is available in three forms: The chlorine will react with organic. when chlorine enters water, it immediately begins to react with compounds found in the water. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. the dose can be determined by: chlorine. Chlorine In Water Equation.
From stock.adobe.com
Lewis structural formula of chlorine, molecular formula Stock Vector Chlorine In Water Equation Includes kit list and safety instructions. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. the dose can be determined by: when chlorine enters water, it immediately begins to react with compounds found in the water. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach).. Chlorine In Water Equation.
From techpools.es
Calculating Intellichems Chlorine Values Chlorine In Water Equation revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. chlorine is available in three forms: Includes kit list and safety instructions. Chlorine dose, mg/l = chlorine. Chlorine In Water Equation.
From socratic.org
What is the chemical equation for HCl dissolving into water and Chlorine In Water Equation generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. the dose can be determined by: Includes kit list and safety instructions. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f.. Chlorine In Water Equation.
From www.youtube.com
AQA Further Reactions of Chlorine YouTube Chlorine In Water Equation chlorine is available in three forms: Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. Includes kit list and safety instructions. when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. The chlorine will react. Chlorine In Water Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the reaction of Chlorine In Water Equation revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. the dose can be determined by: chlorine is available in three forms: Chlorine dose, mg/l = chlorine demand, mg/l +. Chlorine In Water Equation.
From www.alamy.com
Hypochlorous acid (HClO) disinfectant molecule. Formed when chlorine is Chlorine In Water Equation Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. The chlorine will react with organic. chlorine is available in three forms: generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written. Chlorine In Water Equation.
From www.shutterstock.com
Formula And Structure Of Chlorine Dioxide Molecule (Clo2)Main Chlorine In Water Equation Includes kit list and safety instructions. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. the dose can be determined by: Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). chlorine is. Chlorine In Water Equation.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine In Water Equation chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. the dose can be determined by: Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f.. Chlorine In Water Equation.
From www.chemistryviews.org
The Chemistry of Pools ChemistryViews Chlorine In Water Equation Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. chlorine is available in three forms: Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). generate chlorine gas on. Chlorine In Water Equation.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Chlorine In Water Equation Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. generate chlorine gas on a microscale and investigate its reactions. Chlorine In Water Equation.
From www.alamy.com
Chlorine dioxide (ClO2) molecule. Used in pulp bleaching and for Chlorine In Water Equation generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is only slightly soluble in water, with its maximum solubility occurring. Chlorine In Water Equation.
From www.youtube.com
Water Distribution Math Chlorine Demand YouTube Chlorine In Water Equation Chlorine dose, mg/l = chlorine demand, mg/l + chlorine residual, mg/l. The chlorine will react with organic. when chlorine enters water, it immediately begins to react with compounds found in the water. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they won’t cause problems in the. . Chlorine In Water Equation.
From www.numerade.com
SOLVEDElemental chlorine is commonly used to kill in Chlorine In Water Equation The chlorine will react with organic. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. chlorine is added to raw water to eliminate algae and other. Chlorine In Water Equation.
From www.slideserve.com
PPT Chlorine Chemistry PowerPoint Presentation, free download ID Chlorine In Water Equation revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). The chlorine will react with organic. chlorine is added to raw water to eliminate algae and other forms of aquatic life from the water so they. Chlorine In Water Equation.
From www.numerade.com
Chlorine forms from the reaction of hydrochloric acid with manganese(IV Chlorine In Water Equation chlorine is only slightly soluble in water, with its maximum solubility occurring at 49° f. generate chlorine gas on a microscale and investigate its reactions with water or halide ions in this class practical. Gaseous chlorine (100% pure), calcium hypochlorite (hth) and sodium hypochlorite (bleach). chlorine is added to raw water to eliminate algae and other forms. Chlorine In Water Equation.