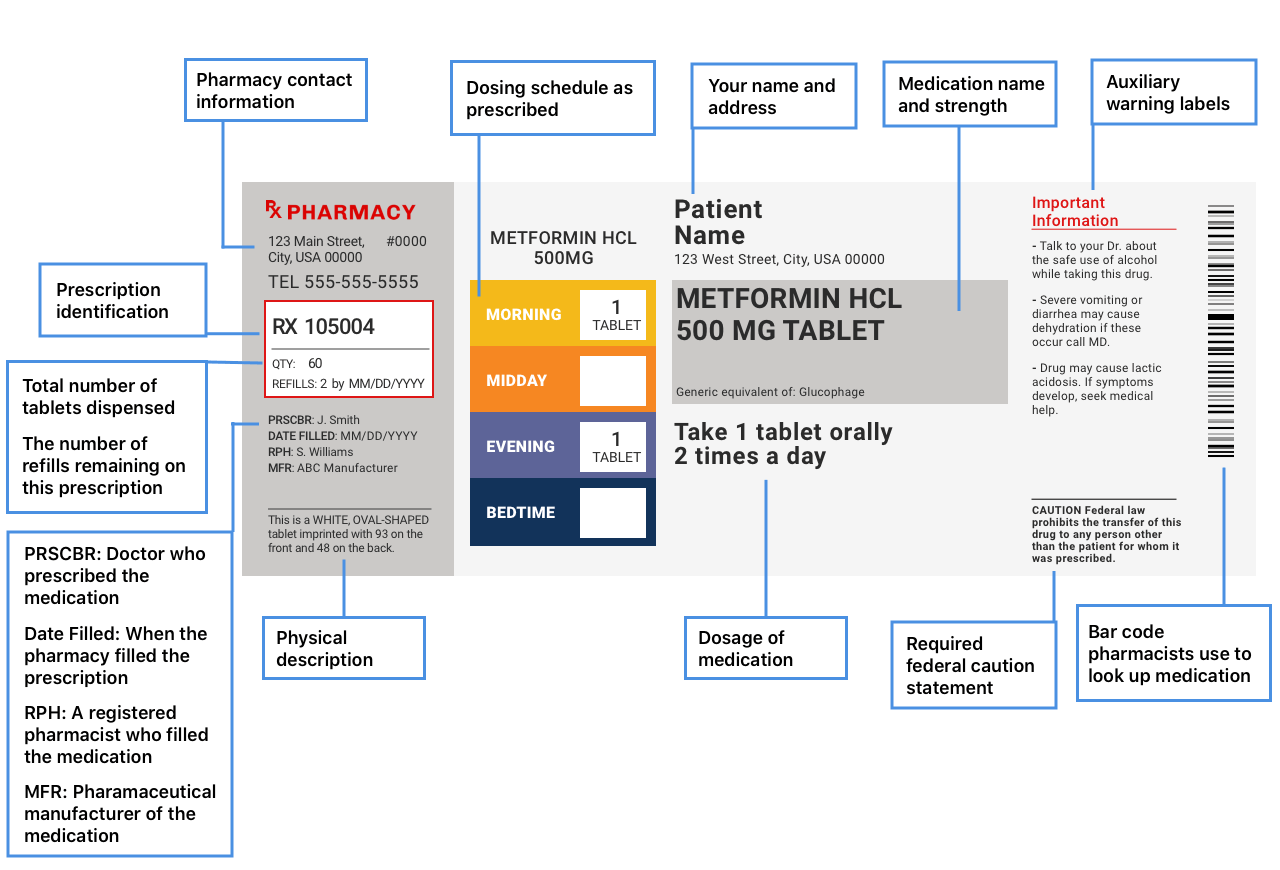
Fda Drug Facts Label Guidelines . This paragraph applies only to prescription. (d) labeling requirements for new and more recently approved prescription drug products. in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Fda required changes to the. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Section 201.66 standard labeling format a. The drug facts labeling is. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl).
from www.drugwatch.com
in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). The drug facts labeling is. (d) labeling requirements for new and more recently approved prescription drug products. Section 201.66 standard labeling format a. This paragraph applies only to prescription. Fda required changes to the. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product.
How to Read OvertheCounter and Prescription Drug Labels
Fda Drug Facts Label Guidelines (d) labeling requirements for new and more recently approved prescription drug products. Fda required changes to the. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. This paragraph applies only to prescription. The drug facts labeling is. Section 201.66 standard labeling format a. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). (d) labeling requirements for new and more recently approved prescription drug products.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. The drug facts labeling is. in 2016, the u.s. in the interest of uniformity of presentation, fda strongly reccommends that the drug. Fda Drug Facts Label Guidelines.
From www.bakingbusiness.com
New FDA campaign educates consumers on new Nutrition Facts Label 2020 Fda Drug Facts Label Guidelines This paragraph applies only to prescription. The drug facts labeling is. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Section 201.66 standard labeling format a. the. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines (d) labeling requirements for new and more recently approved prescription drug products. in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. The drug facts labeling is. This paragraph applies only to prescription. Section 201.66 standard labeling format a. Fda required changes to the. in the. Fda Drug Facts Label Guidelines.
From myoldmeds.com
Read the Label Understanding the Drug Facts Label on Over The Counter Fda Drug Facts Label Guidelines Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. (d) labeling requirements for new and more recently approved prescription drug products. in 2016, the u.s. This paragraph applies only to prescription. The drug facts labeling is. the drug facts labeling for otc drug products is intended to make it. Fda Drug Facts Label Guidelines.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). (d) labeling requirements for new and more recently approved prescription drug products. Fda required changes to the. This paragraph applies only to prescription. Section 201.66 standard labeling format a. Food and drug administration (fda) updated requirements for the nutrition facts. Fda Drug Facts Label Guidelines.
From www.fda.gov
Sample Drug Facts Label FDA Fda Drug Facts Label Guidelines in 2016, the u.s. This paragraph applies only to prescription. Fda required changes to the. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. (d) labeling requirements for new and more recently approved prescription drug products. Section 201.66 standard labeling format a. the nonprescription drug. Fda Drug Facts Label Guidelines.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Drug Facts Label Guidelines (d) labeling requirements for new and more recently approved prescription drug products. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. in 2016, the u.s. Fda required changes to the. This paragraph applies only to prescription. Section 201.66 standard labeling format a. The drug facts labeling is. the nonprescription. Fda Drug Facts Label Guidelines.
From thepointjournal.org
Point 74 “FDA Approved” Let’s Get Real Pat McCarthy The Point Fda Drug Facts Label Guidelines (d) labeling requirements for new and more recently approved prescription drug products. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Fda required changes to the. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling. Fda Drug Facts Label Guidelines.
From pharmapush.com
Drug Facts Labels of US FDA in Easy Language PHARMA PUSH Fda Drug Facts Label Guidelines in 2016, the u.s. (d) labeling requirements for new and more recently approved prescription drug products. Section 201.66 standard labeling format a. The drug facts labeling is. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). the drug facts labeling for otc drug products is intended to. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in 2016, the u.s. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Fda required changes to the. Food and drug administration. Fda Drug Facts Label Guidelines.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label. Fda Drug Facts Label Guidelines.
From www.fda.gov
OTC Drug Facts Label FDA Fda Drug Facts Label Guidelines Fda required changes to the. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the drug facts labeling for otc drug products is intended to make it easier for consumers. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. The drug facts labeling is. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the drug facts labeling for otc drug products is intended to make. Fda Drug Facts Label Guidelines.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Section 201.66 standard labeling format a. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Fda required changes to the. in 2016, the u.s. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Section 201.66 standard labeling format a. The drug facts. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Fda required changes to the. This paragraph applies only to prescription. The drug facts labeling is. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). the drug. Fda Drug Facts Label Guidelines.
From www.lifealert.org
OvertheCounter Medicine Label Fda Drug Facts Label Guidelines This paragraph applies only to prescription. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). The drug facts labeling is. Fda required changes to the. in 2016, the u.s. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Fda required changes to the. (d) labeling requirements for new and more recently approved prescription drug products. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented. Fda Drug Facts Label Guidelines.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Drug Facts Label Guidelines the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Section 201.66 standard labeling format a. in 2016, the u.s. This paragraph applies only to prescription. The drug facts labeling is. Fda required changes to the. in the interest of uniformity of presentation, fda. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). This paragraph applies only to prescription. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Food and drug administration (fda). Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines The drug facts labeling is. This paragraph applies only to prescription. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). (d) labeling requirements for new and more recently approved prescription drug products. Fda required changes to the. Section 201.66 standard labeling format a. the drug facts labeling for. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. (d) labeling requirements for new and more recently approved prescription drug products. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the drug facts labeling. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Section 201.66 standard labeling format a. The drug facts labeling is. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines in 2016, the u.s. The drug facts labeling is. Fda required changes to the. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Section 201.66 standard labeling format a. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc. Fda Drug Facts Label Guidelines.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Drug Facts Label Guidelines The drug facts labeling is. Section 201.66 standard labeling format a. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. This paragraph applies only to prescription. (d) labeling requirements for new and more recently approved prescription drug products. the nonprescription drug facts label. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Fda required changes to the. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. The drug facts labeling is. in the interest of uniformity of presentation, fda. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Fda required changes to the. (d) labeling requirements for new and more recently approved prescription drug products. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Section 201.66 standard labeling format a. the drug facts labeling for otc drug products is intended to make it easier. Fda Drug Facts Label Guidelines.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Drug Facts Label Guidelines Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product.. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Fda required changes to the. in 2016, the u.s. the drug facts labeling for otc drug products is. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. in 2016, the u.s. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. This paragraph applies only to prescription. The drug facts labeling is. the drug facts labeling for otc drug products is intended to make it easier for consumers. Fda Drug Facts Label Guidelines.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Section 201.66 standard labeling format a. This paragraph applies only to prescription. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in the interest of. Fda Drug Facts Label Guidelines.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Fda required changes to the. in 2016, the u.s. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Food and drug administration (fda) updated requirements. Fda Drug Facts Label Guidelines.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. (d) labeling requirements for new and more recently approved prescription drug products. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). This paragraph applies only to prescription. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines This paragraph applies only to prescription. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Section 201.66 standard labeling format a. Fda required changes to the. The drug facts labeling is. the drug facts labeling for otc drug products is intended to make it easier for consumers to read. Fda Drug Facts Label Guidelines.
From www.youtube.com
DRUG FACTS LABELS OF US FDA ll DPHARMA PART 2 ASSIGNMENT ll Fda Drug Facts Label Guidelines The drug facts labeling is. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Fda required changes to the. (d) labeling requirements for new and more recently approved prescription drug. Fda Drug Facts Label Guidelines.